Sol-Gel Transition and Critical Gel in Vulcanization Reaction
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
IThis study highlights how the transition from uncured to cured state in rubber is characterized by the formation of a particular network structure, so-called critical gel. Critical gel is formed in the first stages of vulcanization and is independent by the macromolecular structure and crosslinking.
Keywords: Curing cycle design; Under-cure phase; Spongelike region; Blowing point; Shrinkage; Critical Gel time; Elastomer; Compound; Viscoelasticity
Introduction
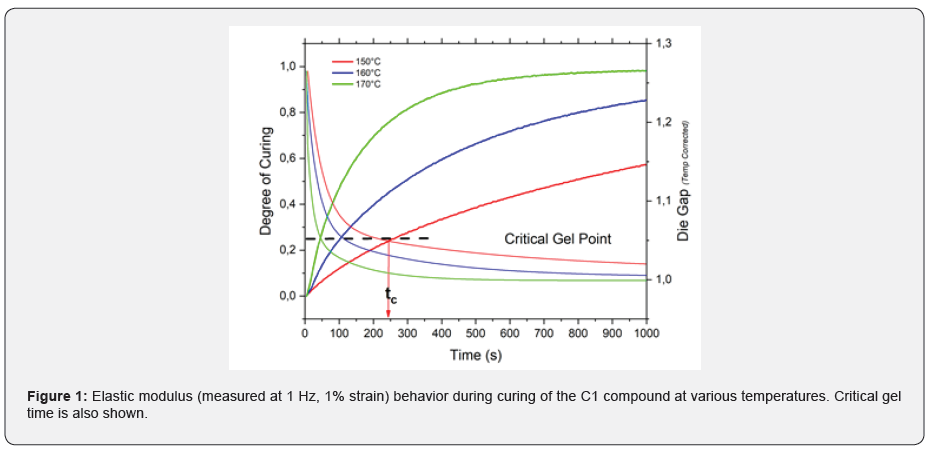
Vulcanization proceeds through three different stages, as shown in Figure 1. The first stage is characterized by an induction phase in which the reactions between the curatives and macromolecules of the polymeric matrix take place. In the second, commonly known as curing stage, cross-linking reactions between the macromolecular chains take place [1-2]. The initial sol-gel transition is characterized by the formation of a “critical gel” in which each polymer chain is chemically linked to at least another one. The reaction then proceeds until the full vulcanization is reached. In the final stage, so-called over-curing reactions take place and they can cause the partial destruction of the network. This phenomenon is called reversion and, in this case, a partial loss of product performance generally occurs [3].
Keywords: The Sol-Gel Transition & Volumetric Contraction
Rheology is commonly used to study the behaviour of phase transitions in polymeric systems [4]. The formation of the network during vulcanization, in which the transition from a rubbery to a solid state takes place, is characterized by the presence of permanent cross-links and by a sol-gel transition with the formation of a critical gel [5-6]. Critical gel shows a relaxation modulus, G(t) described by a power law, as follows:
G (t ) = S.t -n
where parameters S and n represent the rigidity and the critical relaxation exponent of the gel. While it is difficult to measure the G(t) modulus from step shear strain (stress-relaxation) tests, it is much easier to obtain it from simple frequency sweep tests. Applying the Laplace transform [7] of the function describing G(t), we can immediately obtain the complex modulus, G*, as follows:
G* = sL (G (s)) = S.G(1- n).sn
where s= î ω and î is the imaginary unit and ω the frequency. Noticing that G*=G’+ î G’’ the equation 3 is obtained:
Experimental
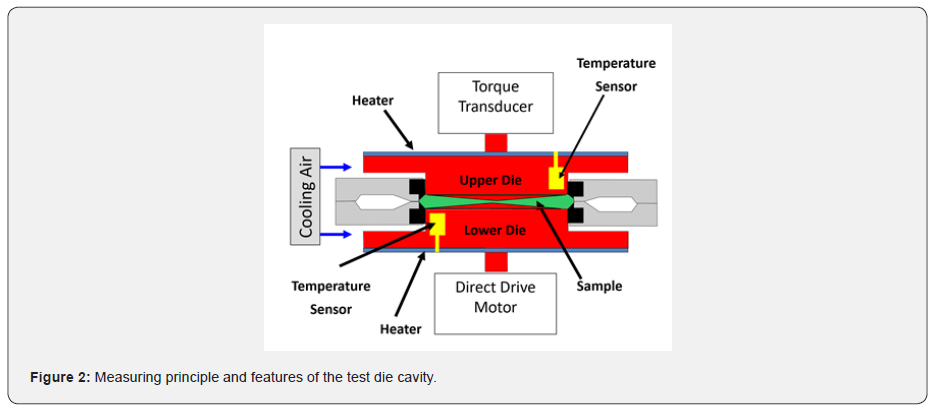
In this study we investigated three different compounds named C1 C2 and C3. C1 and C2 have the same vulcanizing system, based on dicumyl peroxide (DCP) at 1.3 phr (taken as pure), without the addition of fillers. C1 is high cis polyisoprene (IR0310 Kraton) and C2 is NBR nitrile rubber (N3345). C3 is a typical tread rubber compound recipe for truck tire application, with NR, BR and e-SBR as given in Table 1. The elastic modulus G’ (MPa) was measured during isothermal vulcanization at 3 different temperatures, 150°C, 160°C, and 170°C, at a frequency of 1 Hz at 1% of deformation. Simultaneously the volumetric shrinkage of the compound under isothermal vulcanization conditions. The studies were made using a Premier™ RPA Plus analyser, commercialized by Alpha Technologies (company of the Roper Technologies group, Hudson, OH, USA). The resulting torque is measured and through the appropriate built-in data treatment, split into real and imaginary components in order to yield dynamic viscoelastic data, such as G’ and G”. The testing part consists in a biconical test chamber with grooved dies to avoid slippage. The cavity is closed through the action of a ram [8-9]. The torque measuring system is attached to the upper die and a direct drive motor move the lower die sinusoidally over a wide range of strains and frequencies as shown in Figure 2. The critical gel point, that is the time at which the sol-gel transition takes place at a given temperature, has been determined by carrying out a continuous sequence of frequency sweep tests (in the range between 1 and 30Hz) at a given curing temperature.

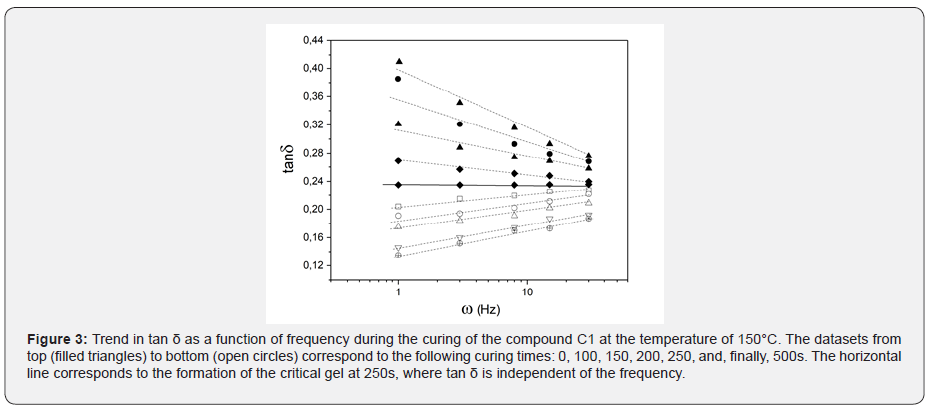
Figure 3 shows the results of these frequency sweep measurement obtained for C1 (IR compound) during isothermal vulcanization at 150°C. Data shows that at 250s, the formation of the critical gel is observed: at the critical gel point tand is no frequency independent as given in eq. 3. As shown inf Figure 1 the time in which the sol-gel transition takes place (tc, time in which critical gel formation is observed), it is characterized by a degree of vulcanization of 0.25-0.30, regardless of the temperature at which the curing reaction occurs. The formation of the critical gel can therefore be related to the development of a crosslinked structure during vulcanization and its dependence on the curing temperature. Figure 3 shows the dependence of the critical gel time on the reciprocal of the vulcanization temperature for all compounds analysed. As expected, the formation of the critical gel occurs faster at higher temperatures, where the rate of vulcanization is faster. The dependence between the critical gel time and the vulcanization temperature follows the Arrhenius' equation as shown by the linear behaviour of in Figure 4. The calculated activation energy values are in excellent agreement with the experimental data available in the literature [5-6].
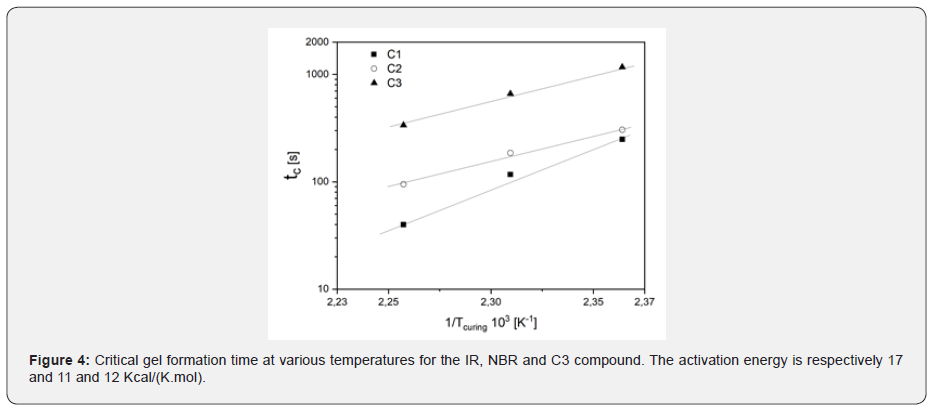
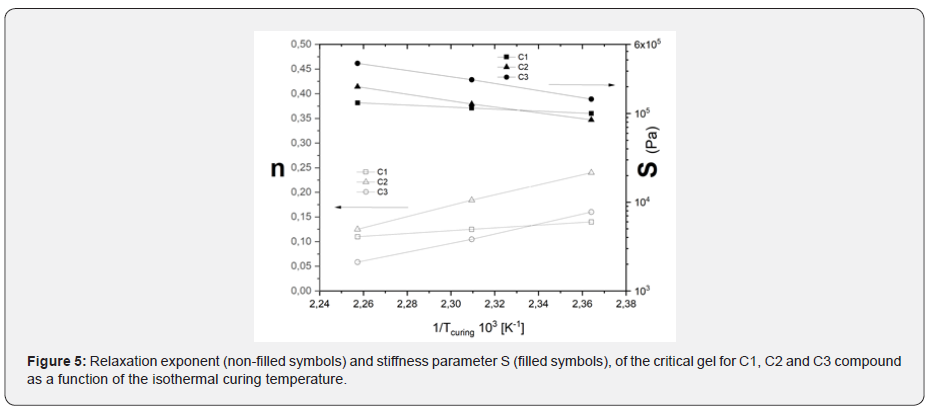
The time to reach the sol-gel transition is faster for the C1 (IR) than for the nitrile compound (C2), while it becomes practically equal at approximately 135°C, which is the temperature at which the DCP reaction rate becomes very slow. For the sulphur cured compound C3, the critical gel time is significantly longer than C1 and C2 at the same temperature. In other words, the same tc has been reached for C3 at a temperature 20°C higher than the peroxide cured compounds. Figure 5, shows the relaxation exponent vs. critical gel stiffness as a function of temperature. It is interesting to note that the stiffness of the critical gel slightly depends on temperature, and is characterized by particularly low values of n, especially at high temperatures (in the order of 0.10). These results suggest that the critical gel structure is qualitatively similar, independently of the elastomers and of the curing system and tend to be stiffer when formed in a curing reaction at high temperature, showing a quite limited time dependent relaxation modulus G(t).
Results and Conclusion
The formation of the network during vulcanization, in which the transition from a rubbery to a solid state takes place, is characterized by a sol-gel transition with the formation of a critical gel. Frequency sweep test has been used to determine the time require to form the critical gel during vulcanization and has been found to takes place at a degree of vulcanization of 0.25-0.30, regardless of the temperature at which the curing reaction occurs. In this paper, it has been also shown experimentally that critical gel time follows the Arrhenius' equation and the stiffness of the critical gel slightly depends on temperature, and is characterized by particularly low values of n, especially at high temperatures (in the order of 0.10). These results suggest that the critical gel structure is qualitatively similar, independently of the elastomers and of the curatives.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
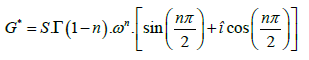


Comments
Post a Comment