Biomimetic Coating on Polymeric Implant Surfaces -The Need of a Quality Standard
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Polyetheretherketone (PEEK) is a promising implant material because of its excellent mechanical characteristics. Although this polymer is a standard material in spinal applications, PEEK has the disadvantage by its relative bio-inertness [1]. This is the reason why coating technologies have built an impressive catalogue of success in many different applications: With a growing need for coating technologies to functionalize the surface of polymeric medical devices, the medical industry saw enormous growth in coating application onto medical devices. Various types of coatings technologies, coating materials and substances are available to date: Spanning from plasma spray coating technologies to dip coating techniques, from titanium or hydroxyapatite, all which enhance cell attachment onto orthopedic implants. But there are also various risks associated with the materials and methods mentioned above: amongst others, delamination, wear debris, abrasion, particle migration, infection or corrosion. Performing an exhaustive literature research and during different test set-ups simulating the predictable way of use of coated polymeric implants the authors have analyzed and evaluated the most common used and regulatory cleared coating materials and technologies in medical-device-applications. This paper is meant to give insights into the safety and performance characteristics of the most commonly used coating materials and methods onto polymeric implant surfaces.
Analyzing safety and performance characteristics under simulated use
Almost all commonly used coating materials and technologies suffer from debris, delimitation and abrasion. Coating technologies incorporating titanium additionally are suspected to corrode, particularly in acidic environments and to cause inflammatory reactions [2-5]. The table below summarizes the different disadvantages and drawbacks of commonly known and regulatory cleared coating technologies. The results presented here have been analyzed and evaluated by Pubmed literature searches and/ or during simulated use tests performed by the authors and/or affiliated companies and institutes (Tables 1 & 2) (Figures 1-3).

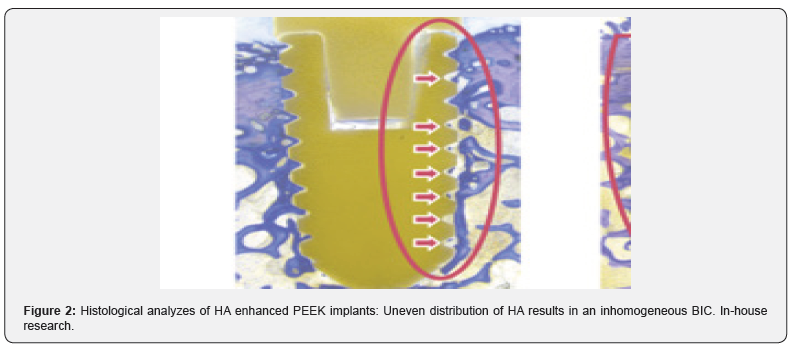
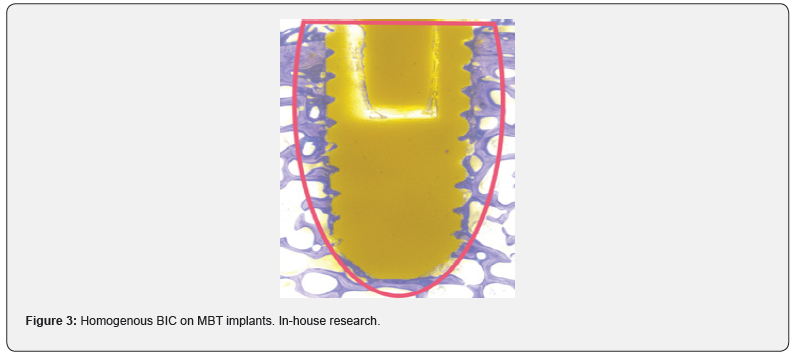
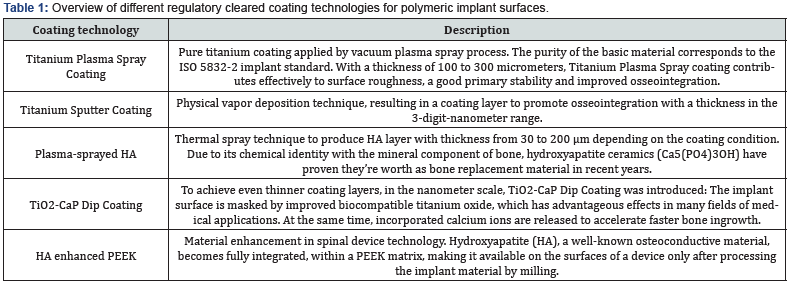
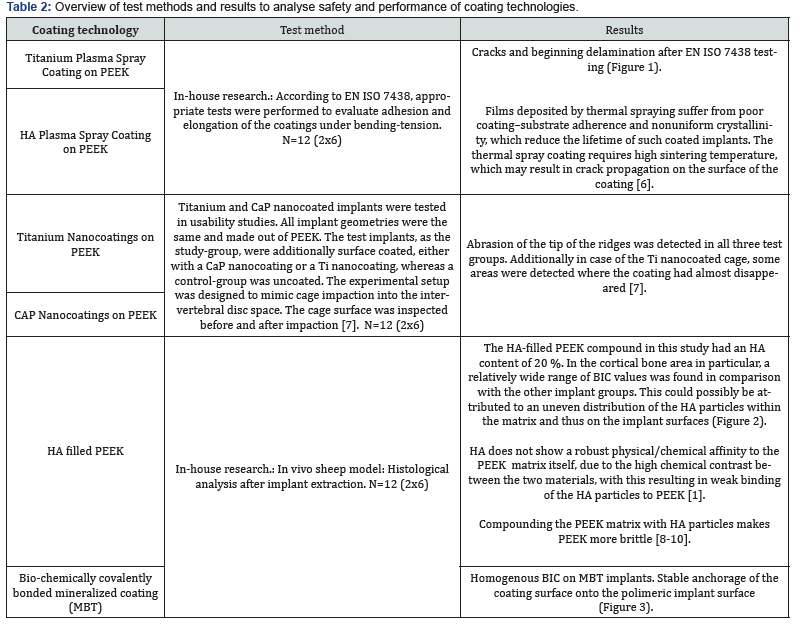
Conclusion
In vitro studies of the above mentioned coating technologies have shown encouraging results regarding osseointegration. But mechanical tests have shown the disadvantages of plasma sprayed coated surfaces, whereas coatings that incorporate titanium may corrode and result in inflammatory reactions. Coatings with a layer-thickness in a 3-digit-micrometer range may influence negatively the engineered topography of 3D-printed implants. With all these different materials and substrates used in coating applications as also due to the various coating technologies, one can get easily lost in a jungle of information: On the one hand side there are some standardized test methods to proof the mechanical stability of the coating layers on the implants surface, on the other hand these methods cannot be used to characterize the mechanical behavior of the various technologies. For biocompatibility testing different set-ups of in-vitro cell test methods and in-vivo animal models can be found in literature. The different coating materials can easily lead to a higher risk-classification of the coated implant. Different and unstructured testing methods and untransparent information may lead to wrong decisions, resulting in reduced safety and performance of polymer-implants. The authors suggest to define and establish an evaluation matrix for different surface functionalization technologies and to introduce a quality standard for coating technologies with transparent safety and performance parameters. To introduce such a quality standard will be subject of further research work, guidelines and publications.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php


Comments
Post a Comment