Major Reduction in Chemical Curatives for Rubber Articles-Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE

Opinion
The sulfur cure system in the
ethylene-propylene-diene (EPDM)-based Curtain Wall Seal (CWS) has two
accelerators, adding up to 2.75 parts per hundred rubber (phr) by
weight, and two activators (ZnO: 5phr, stearic acid:1phr) [1]. In total,
8.75phr chemicals are used to fully cure the article with 1phr
elemental sulfur. In rubber formulations, chemical curatives are
indispensable and once reacted with sulfur at elevated temperature, they
produce crosslinks between the rubber chains and provide shape
stability, which is essential for the performance, durability and life
of the final product in service. Excessive use of chemical curatives is
harmful to health, safety, and the environment. According to the
European Directive 67/548/EEC, chemicals such as sulfenamide
accelerators, zinc oxide and stearic acid are very toxic to aquatic
organisms [2]. Stearic acid causes skin and eye irritation in human and
is classified as highly flammable [3]. These chemicals are used
extensively in the sulfur vulcanization of a wide range of EPDM-based
rubber articles.
Keywords:
Ethylene-Propylene-Diene rubber; N-tert-butyl-2-benzothiazole
sulfenamide; Zinc oxide; Stearic Acid; Vulcanization; Oscillating Disc
Rheometer
Aims & Objectives
The aim of this study was to significantly reduce use
of N-tert-butyl-2-benzothiazole sulfenamide (a fast curing delayed
action accelerator, Santocure TBBS) and zinc oxide (primary activator)
in the sulfur cure systems of EPDM-based Curtain Wall Seal and eliminate
stearic acid (secondary activator) entirely from the cure system. A new
method for measuring the exact optimum amount of the chemical curatives
required in the sulfur vulcanization of EPDM rubber was used [4]. All
the cure tests were performed at 160oC in an oscillating disc rheometer
(ODR) curemeter to produce cure traces from which scorch and optimum
cure times, cure rate index and minimum and maximum torques were
measured.
Experimental

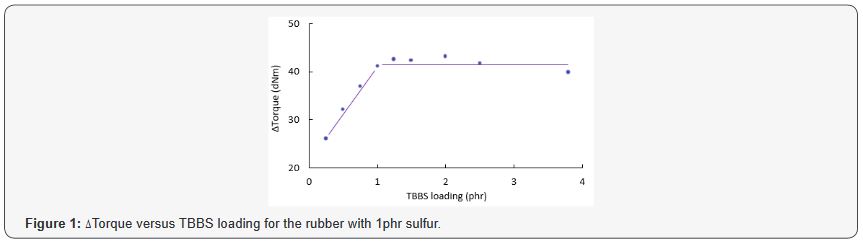
Figure 1 demonstrates Δtorque versus TBBS loading for
the EPDM rubber with 1phr sulfur. ΔTorque, which is the difference
between the maximum and minimum torques on the cure trace of the rubber
and is an indirect indication of crosslink density changes in the
rubber, increased steeply from 26 to 42 dNm as the loading of TBBS was
boosted from 0.25 to 1phr. Subsequently, there was no improvement in
Δtorque once the amount of TBBS reached 3.8phr. Apparently, 1phr TBBS
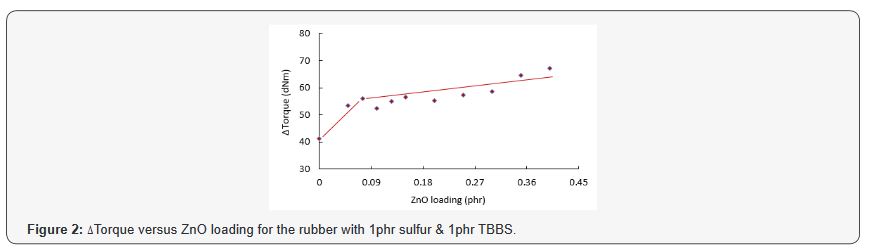
was enough to react the sulfur with the rubber. Zinc oxide was then
added to improve the efficiency of TBBS. ΔTorque rose noticeably from 41
dNm at 0 phr ZnO to 56 dNm at 0.075phr ZnO and the rate of increase
slowed down significantly thereafter. Δtorque then reached to about 67
dNm when the loading of ZnO was raised by an additional 0.325phr (Figure
2). It is interesting that a small amount of ZnO, i.e. as low as
0.075phr, had such a major influence on the performance
of TBBS in the cure system as indicated by a significant rise
in Δtorque. When 0.5phr stearic acid was mixed with the EPDM
rubber with 1phr sulfur, 1phr TBBS & 0.075phr ZnO, torque decreased
from 56 to 47 dNm. torque subsequently continued decreasing
slowly to about 42 dNm when the loading of stearic acid
was raised to 2.5phr (Figure 3). Evidently, the crosslink density as
indicated by Δtorque did not benefit from the addition of stearic
acid to the rubber. Moreover, the scorch and optimum cure times
increased, and the rate of cure as indicated by the cure rate index
declined noticeably when stearic added was added [4]. Consequently,
stearic acid was eliminated from the cure system entirely.
In the absence of stearic acid, no zinc stearate was formed in the
rubber. Hence, zinc stearate is not an essential ingredient in the
curing of rubber as has been claimed [5]. The scorch time (ts2) and
the optimum cure time (t95) were 6.2 & 21.5 min, respectively. The
rate of cure as indicated by the cure rate index (CRI) was 6.5 min-1.
Clearly, requirement for the accelerator and primary and secondary
activators to fully cure the rubber at 1phr loading of sulfur is
much lower, i.e. TBBS by 64wt%, zinc oxide by 98.5wt% and stearic
acid by 100wt%, than the amounts currently used in the cure
system of the CWS at the same loading of sulfur. All the indications
are that a significant decrease in the loading of the chemical curatives
in the cure system has no adverse effect on the vulcanization
of the article. In fact, cure efficiency improves when less
accelerators and activators are used with sulfur. This method can
be applied to reduce excessive amount of the chemical curatives
in other industrial rubber articles.


For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com


Comments
Post a Comment