Gellan Gum Immobilized Anticancer Drugs and Gold Nanoparticles in Nanomedicine-Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
This review is devoted to recent progress in the
design of anticancer drug delivery systems with participation of unique
polysaccharide gellan gum. At first a brief literature survey on
conformational and phase behavior of gellan gum as a function of
external stimuli, such as temperature, pH, salt addition etc. is
presented. Then the immobilization protocol of anticancer drugs and gold
nanoparticles within gellan-based hydrogel matrix is discussed. Release
of anticancer drugs from gellan gel matrix to outer solution is
considered. Cytotoxicity of gellan gum-immobilized gold nanoparticles
together with their anticancer activity is summarized.
Keywords: Gellan gum; Coil-helix transition; Hydrogel; Anticancer drugs; Gold nanoparticles; Cytotoxicity; Anticancer activity
Abbrevations:
GG: Gellan Gum; EOR: Enhanced Oil Recovery; AgNPs: Silver
Nanoparticles; AuNPs: Gold Nanoparticles, AuNR: Gold Nanorods, DDS: Drug
Delivery System; PCT: Paclitaxel; Ge-Pred NHs: Gellan-Prednisolone
Nanohydrogel; PCT Ge-Pred NHs: Gellan-Prednisolone-Paclitaxel
Nanohydrogel; GG-AuNPs: AuNPs Covered by Gellan Gum; GG-AgNPs: AgNPs
Covered by Gellan Gum; DOX: Doxorubicin Hydrochloride; SL: Sophorolipid;
NIR: Near-IR; PPTT: Plasmonic Photothermal Therapy; CTAB:
Cetyltrimethylammonium Bromide; LBL: Layer-By-Layer; SaOS-2: Sarcoma
Osteogenic; PVCL: Poly(vinylcaprolactame); TNBC: Triple Negative Breast
Cancer
Introduction
Over the past few decades, microbial polysaccharides
have been under intense investigation due to their advantageous
physicochemical properties. Currently, one of the most widely studied
and comprehensively described member of this group is gellan a linear
polymer produced by Sphingomonas elodea
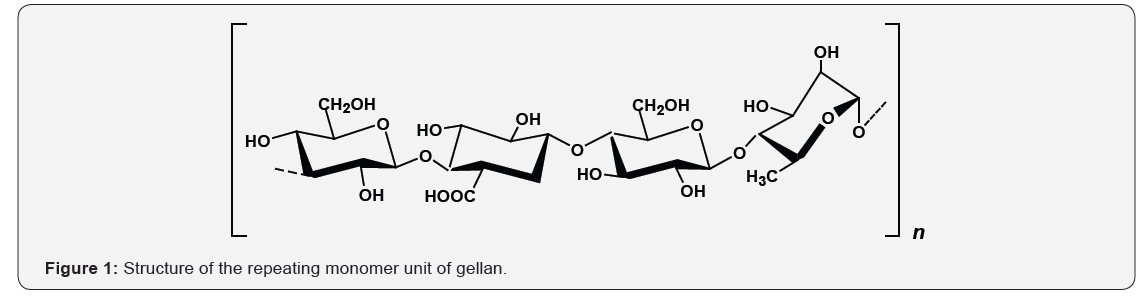
consisting of a tetrasaccharide repeating unit of 1,3-linked
β-D-glucose, 1,4-linked β-D-glucoronic acid, 1,4-linked β-D-glucose, and
1,4-linked α-L-rhamnose [1] (Figure 1). Fermentative production and
manufacturing of gellan on industrial scale is described in reviews
[2,3].
So far most of the studies have been focused on the
application of gellan as a food ingredient. Last year’s however, the
applicability of gellan gum in EOR was demonstrated [4-12]. Due to the
unique structure and beneficial properties, gellan is currently
described as a potent multifunctional additive for various
pharmaceutical products. Specific gelling properties in different media
led to the development of controlled release forms based on gellan.
Various formulations have been studied including oral, ophthalmic, nasal
and other [13,14]. Recent
report [13] suggests that gellan-based materials can also be
used in regenerative medicine, stomatology or gene transfer
technology. Gellan gum-based hydrogels exhibit excellent in vivo
and in vitro biocompatibility [15], tunable physical mechanical
and injectable properties [16-18] for application in regeneration
of cartilage [16,17], tissue engineering [19], cell encapsulation
[20], nucleus pulposes regeneration [21]. Recent progress in the
design of multi-functional hydrogels with participation of gellan
gum in the context of biomedical engineering and regenerative
medicine is discussed and summarized in recent review [22]. In
spite of a wide application of gellan in medicine, pharmacy and
biotechnology it is noteworthy that the gellan based anticancer
formulations have not been described.
Conformational and phase behavior of gellan gum in response to external stimuli
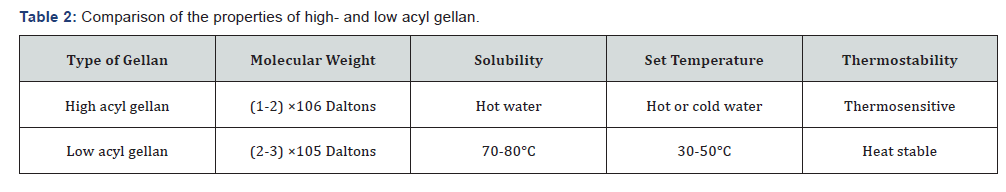
Table 1 represents the chemical and physical properties
of gellan. The native gellan is composed of high acyl and low
acyl precursors [23]. The main difference between them is that
the high acyl gellan contains two acyl substituents: acetyl and
L-glyceril [24]. Low acyl gellan is obtained by removal of the acyl
residues by alkaline hydrolysis [25]. Differences between highand
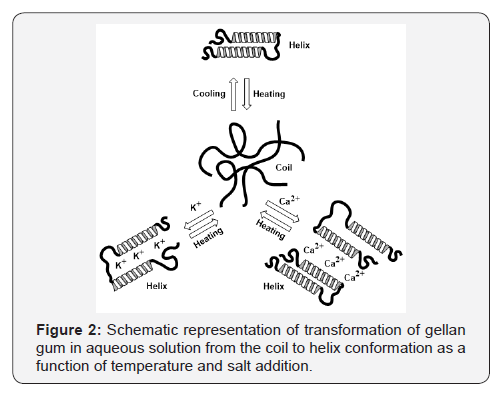
low acyl gellan are summarized in Table 2 [26]. Authors
[1,27] comprehensively reviewed the structure, conformation,
gelation, topology, rheology, and application aspects of gellan.
The coil-helix conformational and sol-gel phase transitions of
gellan gums induced by temperature, salt addition, pH change
etc. became the main subject of many studies [28-33]. A series
of publications cover formation of interpenetrating networks
with participation of gellan and natural polymers [34-42].
It is commonly accepted [43-51] that gellan gum exhibits
a conformational change from the disordered state (single
chain) to the ordered state (double helix) with decreasing of
temperature, and the gelation is considered to be mediated by
the double-helix formation and the association of such helices,
which is enhanced by the presence of mono- and divalent
alkaline and alkaline earth cations [52-55] (Figure 2).
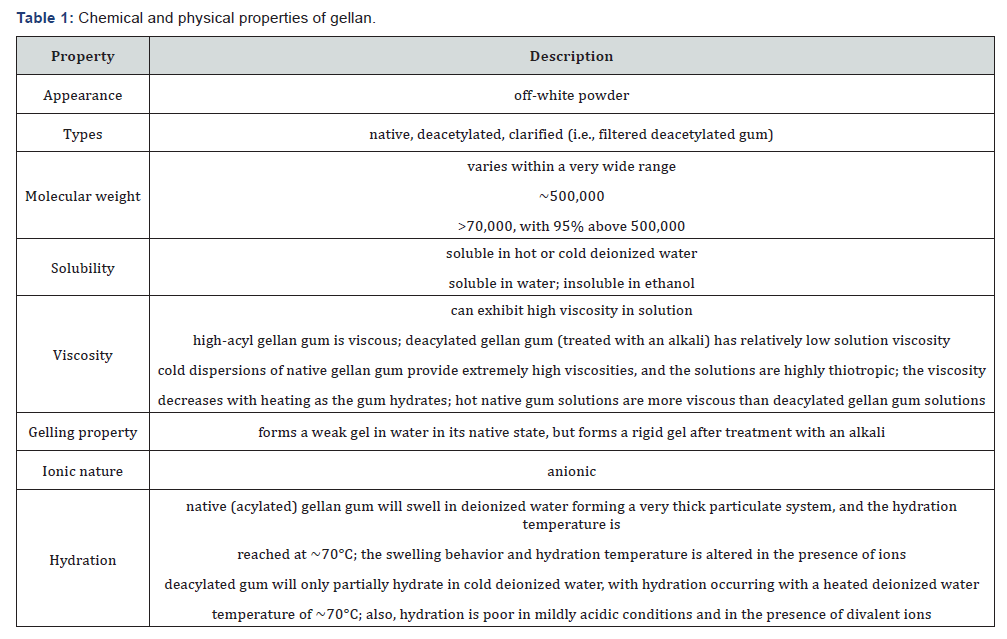
As gellan molecules contain the carboxyl groups in the
repeating unit, the gelation of gellan is remarkably enhanced
by the addition of cations in aqueous solutions. It has been
established that the extent of aggregation and effectiveness in
promoting gel formation by addition of ions follows this order:
Cs+> Rb+> K+>Na+> Li+. This sequence follows the Hofmeister
series and agrees well with increasing of the ionic radius of
cation species. The higher effectiveness of divalent cations in
comparison with monovalent ones may be attributed to additional
crosslinking of gellan chains due to cooperative binding (or
“bridging”) of the divalent cations between glucuronate residues
according to their ionic radii. Divalent cations seem to bind
directly to gellan macromolecules to form aggregates of gellan
helices with the effectiveness of Ca2+˃Mg2+ [56-60]. The main
difference between the monovalent and divalent cations is
that the monovalent cations shield the electrostatic repulsion
between the COO- while the divalent cations, rather than by
suppressing electrostatic repulsion, form interchain ionic bonds
with carboxylic groups of the glucuronic acid units resulting in
the aggregation of the double helices [55,56]. As for the divalent
cations, they do not appear to obey the Hofmeister series and the
order among the divalent cations is more difficult to rationalize.
Gellan salts with the monovalent cation, such as lithium or
potassium, form stiff gels and that with divalent cation, Ca2+,
make a more rigid gel. The mechanism for conformational
change of gellan in the presence of mono- and divalent cations
can be represented as shown in Figure 2 [61]. K+ accelerates
the formation of cooperative hydrogen bonds between gellan
molecules by the charge-shielding effect and hydrogen bonds
reinforces the double-helices and their aggregates. Ca2+ forms
ionic bonds between carboxylic groups of gellan in addition to
hydrogen bonds and leads to the continuous structural change
depending on concentration of the divalent cations.
Immobilization of anticancer drugs within gellan hydrogel matrix
Recently authors [62,63] developed gellan-based
nanohydrogel systems to deliver multiple drugs: prednisolone
and paclitaxel. Prednisolone was chemically linked to the
carboxylic groups of gellan while placitaxel was physically
entrapped into gel marix. The synergistic anti-inflammatory and
anti-cancer effect were reached with respect to malignant cells
and tumor inflammatory components.
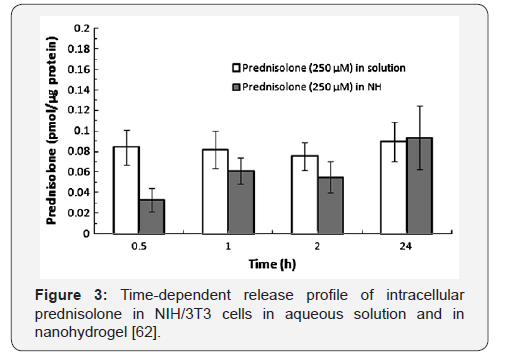
The kinetics of prednisolone release from gellan hydrogel
was measured with respect to NIH/3T3 cells. Figure 3 shows the
intracellular release kinetics of gellan-immobilized prednisolone
from the gel matrix. It is seen that 40% of total prednisolone is
delivered during 30min, 70% after 1h and 100% drug delivery
after 24h. It is suggested that the release of prednisolone from
gellan-based nanohydrogel is hydrolysis of ester bonds between
prednisolone and gellan gum by esterase. Immobilized within
gellan nanohydrogel prednisolone exhibits a core-shell structure
and allows solubilizing up to 40% water-insoluble drug paclitaxel
in hydrophobic environment. The main role of paclitaxel is
disrupting of the dynamic equilibrium within the microtubule
system and inhibiting the cell replication. The cell-killing drug
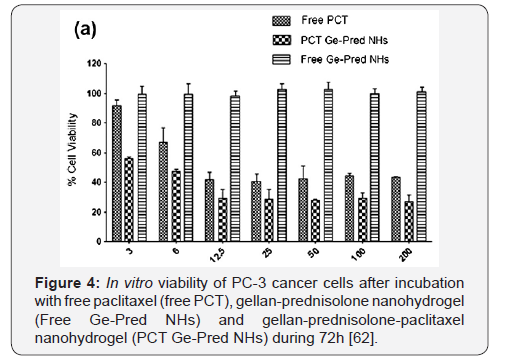
formulation consisted of gellan-immobilized prednisolone with
loaded paclitaxel. The latter released from gellan-prednisolone
nanohydrogel kills the cancer cells with higher efficiency (56.1
0.8% cell viability) than free drug (91.6 3.5 cell viability)
especially at lower concentration (3nM) (Figure 4).
The cell killing effect of gellan-prednisolone-paclitaxel
nanohydrogel was also tested with respect to A2780, MDAMB-
231 and Skov-3 cells. Thus gellan-immobilized antiinflammatory
and anti-cancer drugs can be effective for
treatment of malignant and inflammatory cells involved into
tumor microenvironment. Analgesic, antipyretic and antiflammatory
drug-diclofenac sodium was immobilized into
the matrix of polymethacrylamide-grafted-gellan gum and its
sustained in vitro release kinetics was studied [64]. It was shown
that the diclofenac sodium releases over a period of 8 h and the
release profile is described by Higuchi square root kinetic model
and release mechanism is governed by Fickian diffusion.
Gellan gum immobilized gold nanoparticles for treatment of cancer cells
It is well known that cancer is one of the leading causes
of mortality in the modern world, with more than 10 million
new cases every year. Targeting nanoparticles that selectively
recognize and destroy cancer cells in the body remain key
concept in nanomedicine [65-67]. According to literature survey
of authors [68] only 7 out of 1000 administered nanoparticles are
applicable in a mouse model limiting their clinical translation.
Authors [69] concisely highlighted the current state and recent
advances of stimuli-responsive polymers commonly employed
in oncology applications.
Gold nanoparticles (AuNPs) with controlled geometrical,
optical, and surface-chemical properties are the priority
research of intensive studies and applications in cancer
diagnosis, treatment and as drug delivery system (DDS) [70].
The effectiveness of many anticancer drugs is limited due to
the inability to reach the target site in sufficient concentrations
and efficiently exert the pharmacological effect without causing
irreversible unwanted injury to healthy tissues and cells. The
cellular uptake and toxicity of AuNPs stabilized by gellan gum
(GG-AuNPs) was studied on mouse embryonic fibroblast cells,
NIH 3T3 and human glioma cell line LN-229 [71]. It was shown
that in the cancerous cells the GG-AuNPs were localized mainly
in the cytoplasm and perinuclear region of the cells. Oral
administration of GG-AuNPs did not cause any toxicity in rats for
28 days and was no any significant difference in hematological,
biochemical and histopathology of organs demonstrating
potential of GG-AuNPS as DDS.
The AuNPs stabilized by gellan gum was loaded by
doxorubicin hydrochloride (DOX) one of the potential and wellknown
anticancer drugs [72] was conjugated with sophorolipid
(SL) [73] and their cytotoxicity were evaluated with respect to
human glioma cell line LN 229 and human glioma stem cell line
HNGC-2 (Figures 5 & 6).

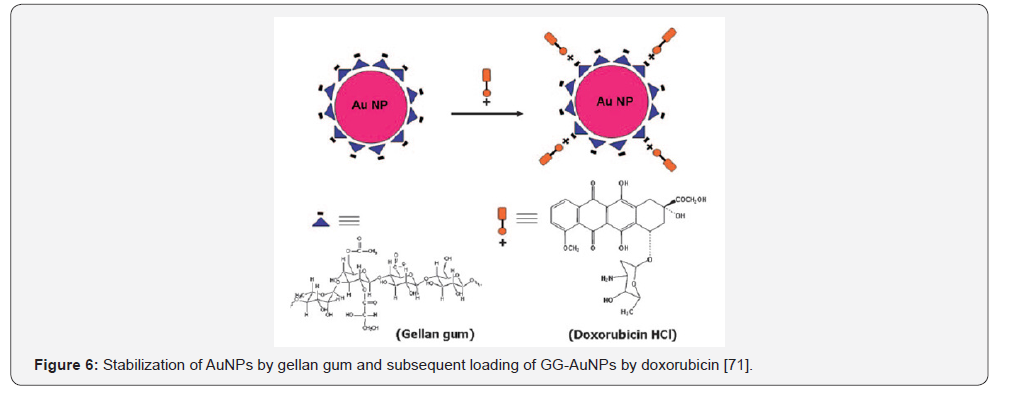
Both SL-conjugated and DOX-loaded gellan gum containing
AuNPs exhibited increased effectiveness against glioma tumors.
The same authors [74] studied the antibacterial activity of the
dispersions of silver nanoparticles (AgNPs) stabilized by gellan
gum (GG-AgNPs), the cytotoxicity of GG-AgNPs against mouse
embryonic fibroplast cells NIH 3T3 and also evaluated the in
vitro diffusion of AgNPs dispersions/gels across rat skin. The
results show that GG capping effectively passivates the AgNPs
and does not display any cytotoxicity against NIH 3T3 and
exhibits eligibility for topical treatments.
Photothermal damage of cells is currently one of the most
promising research avenues in the treatment of cancer and
infectious diseases. The essence of this phenomenon is as
follows: AuNPs have an absorption maximum in the visible or
near-IR (NIR) region and get very hot when irradiated with
corresponding light. If, they are located inside or around the target
cells (which can be achieved by conjugating gold nanoparticles
to antibodies or other molecules), these cells die. The revolution
in thermal cancer therapy is associated with 20-40nm AuNPs
that convert the 20ns laser irradiation (514nm) to local heat (up
to 40-45oC), and selectively kill the cancer cells (Figure 7). This
method called plasmonic photothermal therapy (PPTT) [75] has
extensively been researched and used for biomedical application
[76]. The PPTT has much potential in diagnosis, treatment and
evolution of diseases, in particular cancer [77]. In recent review
[78] the advancements of plasmonic nanoparticles and films in
the field of biomedicine was overviewed.
Among the numerous nanomaterials the best one is gold
nanoparticles (AuNPs) because of their biocompatibility, low
toxicity, ability to absorb in visible or NIR region, excellent
photostability, and availability in various morphologies [79].
Among the gold nanoparticles the gold nanoshells [80] and
nanorods (AuNRs) [81] are especially suitable for PPTT due to
their tunable longitudinal plasmon band in the NIR region [82].
Small spherical AuNPs exhibit poor NIR absorption, therefore
nanoaggregates, nanoshells, nanorods and nanomatryoshkas
stabilized by functional polymers are suitable for PPTT [78].
Gellan gum coated gold nanorods (GG-AuNRs) was fabricated
by authors [83] and used for intracellular drug delivery and
imaging. The preparation strategy of AuNRs includes several
steps: at first the fine dispersed AuNRs is synthesized by a
seed-mediated growth method using cationic surfactant -
cetyltrimethylammonium bromide (CTAB) as surface passivant
[84], then the layer-by-layer (LBL) technique is used for coating,
and finally AuNRs are coated by gellan gum (Figure 8).
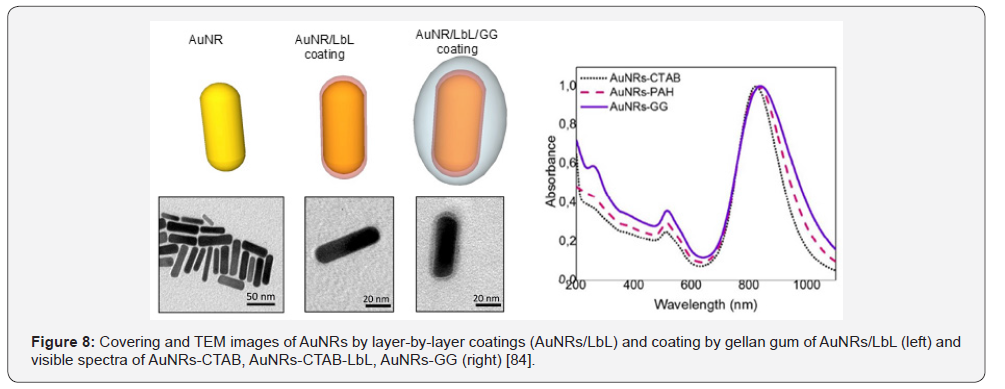
The direct use of as-prepared AuNRs with biological
materials is highly limited because the cytotoxicity of CTAB is
high and can lead to cell death. The successive deposition of poly
(acrylic acid), poly (allylamine hydrochloride) and GG allows the formation of GG shell with nanometric size around individual
AuNRs. The cytotoxicity and osteogenic ability of gellan-coated
AuNRs was tested with respect to SaOS-2 (Sarcoma osteogenic),
a human osteoblast-like cell line commonly used as osteoblastic
model [85]. It was found that AuNR-GG were not cytotoxic after
14 days of culturing and were localized inside lysosomes. The
images in Figure 9 show that AuNRs-GG is aggregated within
multilammelar vesicles identificed as lysosomes.

NIR lasers are selected due to higher penetration of human
tissue resulting in minimal damage. In vitro experiments show
that heating of tumor tissues is observed in the presence of
NIR-exposed AuNRs, however laser irradiation in the absence of
AuNRs causes negligible damage of healthy tissues [78]. Without
coating by biocompatible polymers, AuNRs cannot infiltrate the
blood vessels and therefore their concentration increases in
plasma. In vivo tumor ablation requires a tissue temperature of
around 48-50oC for successful operation.
Magnetic nanoparticles coated by GG exhibited low
cytotoxicity with potential drug delivery applications [86].
Apart from gellan both natural and synthetic polymers can be
used for stabilization and coating of AuNRs. Absorption spectra
of polymer-coated AuNRs are in NIR region and equal to 770nm
(Figure 10a). According to TEM measurements the average
size of AuNRs covered by poly(vinylcaprolactame) (PVCL) is <
100nm (Figure 10b).
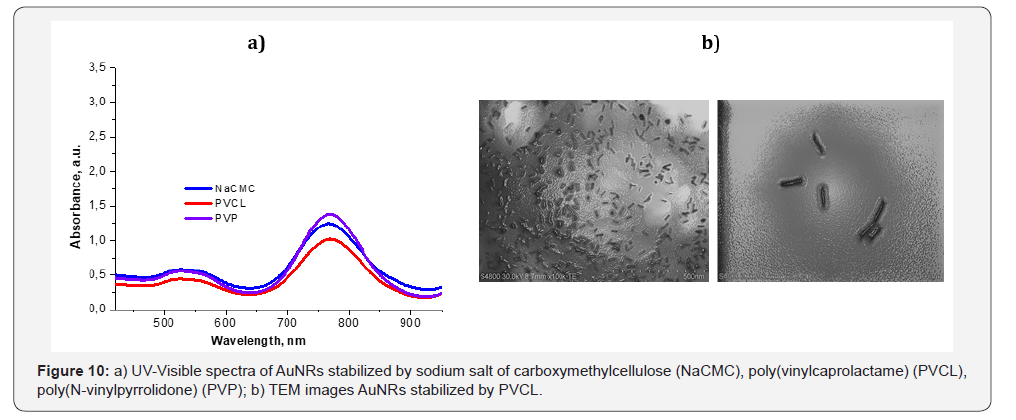
Multilayered Au nanoparticles (Au/SiO2/Au ~ 90nm) called
as nano-matryoshkas (“matryoshka” is Russian nesting doll)
was tested against triple negative breast cancer (TNBC) tumors
[87]. In vivo injection of Au nanomatryoshkas and NIR treatment
(2W.cm-2 for 5min) of TNBC tumor-bearing mice show health
improvement and complete recovery for two months (Figure
11a). In contrast, NIR treatment of TNBC in saline water without
Au nano-matryoshkas considerably increases the size of tumor
for 18 days (Figure 11b).

The Au-based nanomaterials have failed in clinical trials
as PPTT agents. The further development of PPTT and its
acceptance in actual clinical practice depends on success in
solving many problems, the most important ones being
a) The choice of nanoparticles with optimal optical
properties,
b) The enhancement of nanoparticle accumulation in
tumors and the lowering of total potential toxicity, and
c) The development of methods for the delivery of optical
radiation to the targets and the search for alternative
radiation sources combining high permeability with a
possibility of heating AuNPs.
The selection criteria of PPTT depend on
a) The ability of gold nanoparticles to absorb in the near-
IR region;
b) Size of nanoparticles (usually less than 100nm);
c) Low toxicity (in terms of excluding or replacement of
toxic CTAB);
d) Good biocompatibility and easy biodegradability
of polymeric coatings used for entrapment of gold
nanoparticles. Moreover, the aggregated AuNPs should be
disintegrated and removed from the organs and not cause
tissue damage or metal toxicity.
It is expected that in near future the priority research will be
focused on probing the fundamental interactions of nanoparticles
with organs and tissues that accumulate, sequester or eliminate
nanoparticles (such as liver, spleen and kidney), as well as the
interactions between nanoparticles and tumors with respect to
the physico-chemical properties of the nanoparticles.
Conclusion
The unique properties of gellan gum, in particular,
biocompatibility, low toxicity, biodegradability, commercial
availability and low cost argue the successful application of
this class of polysaccharide in biomedicine, pharmacy and bioand
nanotechnology. The ability of gellan to undergo coil-helix
conformational, sol-gel phase transitions, and stimuli-sensitive
character of macromolecules to response temperature, pH,
salt addition, addition of organic ions and molecules open new
perspectives to design drug delivery systems. Anticancer drugs
and gold nanoparticles immobilized within gellan gel matrix
is effective for treatment of cancer cells. It is expected that in
near future the priority research will be focused on probing
the fundamental interactions of nanoparticles with organs and
tissues that accumulate, sequester or eliminate nanoparticles (such as liver, spleen and kidney), as well as the interactions
between nanoparticles and tumors with respect to the physicochemical
properties of the nanoparticles. For the successful
application of nanoparticles there should be a coordinated
research program to establish correlations between the particle
parameters (size, shape, and functionalization with various
molecular probes), the experimental parameters (model; doses;
method and time schedule of administration; observation time;
organs, cells and subcellular structures examined; etc.), and the
observed biological effects.
Acknowledgements
This work was supported by Sichuan Science and Technology
Program (No. 2018HH0024, 2018-2019) and carried out in the
frame of collaborative research project entitled “Fabrication
and controlled drug release of thermosensitive gradient
nanocomposite hydrogels” between College of Chemistry,
Sichuan University, China and Institute of Polymer Materials and
Technology, Kazakhstan.
For more Open Access Journals in Juniper Publishers please
click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please
click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please
click on: https://juniperpublishers.com


Comments
Post a Comment