Research of Hydrogen Absorption-Desorption by Ti-Al-Nb Alloy-Juniper Publishers
Authored
by Kurbanbekov ShR
Abstract
The paper presents kinetics of hydrogen sorption of
Ti-23.5at%Al-21.5at%Nb alloys in isothermal conditions underthe
temperature of 450, 500 and 550 °С. It was determined that maximum
quantity of absorbed hydrogen is observed at material of sorbed alloy
under the temperature of 550 °С. That presents approximately 0.289mass%.
It had been found that hydrogen is released under the temperatures
within 700 …790 °С. In addition, it was revealed that maximum release of
hydrogen composes 85% of samples saturated under the temperature of 550
°С.
Keywords: Intermetallic compound; Absorption-desorption; Hydrogen; Plasticity; CrucibleIntroduction
As is known, to find a safe method for reversible
hydrogen storage is currently one of the important issues in the field
of hydrogen energetics. Storage of the hydrogen in various hydrides of
metals and alloys is one of the advanced methods to solve this issue
[1]. Application of alloys for hydrogen storage and its use depends on
several tasks, which focused on increasing of sorption properties and
cyclic stability of alloys. Ti-Al alloys are one of the efficient
materials for storage of hydrogen [2,3]. Using of alloys to storage
hydrogen and its use depends on several tasks, which are to increase the
sorption properties and cyclic stability of alloys. Alloys based on
Ti-Al are one of the most effective materials for hydrogen storage
[2,3]. It is known that, additional introduction of niobium into the
Ti-Al system significantly increases the plasticity of Ti3Al,
intermetallic which can be explained by a decrease in the degree of
ordering and decrease in the share of covalent bond [4]. Also,
additional introduction of niobium into the Ti-Al system [5,6] leads to
an increase in its absorption-desorption properties of hydrogen due to
the formation of nanoscale phases having less dense packaging compared
to the face centered close-packed lattice of Ti3Al.
The purpose of this paper is to determine the optimal absorption-desorption temperatures of hydrogen to the sample
materials based on Ti-Al-Nb system and to study the changes in its structural-phase state.
Materials and Methods of Research
Ti (99.9%), Nb (99.96%) and Al (99.98) powders were used as initial raw materials for producing Ti-Al-Nb-composite.
Technology of sparkplasma sintering (SPS-technology)
of powder mixtures was used to create compact samples based on
intermetallic Ti-Al-Nb system. Sintering of powder mixtures was
conducted on a special facility Labox-1575. Research of hydrogen
sorption kinetics by intermetallic compounds of Ti-23.5at%Al-21at%Nb
system was conducted on an experimental facility VIKA [7] under the
temperatures of 450, 500 and 550°С. The facility consists of a working
chamber, pumping system and information-measuring system (IMS).
Differential pumping system including forevacuum pump NVR-5DM with a
nitrogen trap and two magnetic discharge pumps NORD-100 and NORD-250 was
used to ensure the required pressure in the working chamber of the
facility. Forevacuum pump is used to pre-pumping of gases from the
working chamber after loading the sample into the crucible, magnetic
discharge pump NORD-250 is used for pumping the working chamber and the
measuring path in the annealing process after loading samples, the pump
NORD-100 is used to create high vacuum in the chamber and the measuring
part of the experimental facility during the experiment. The
experiments consisted the following: Ti-23.5at%Al-21at. %Nb
sample was loaded in a special ampoule device (AD). After
loading of the sample, the high–temperature decontamination
of AD cell with the samples was conducted for 30 minutes at
a temperature of 800-850°C and a constant pumping of the
AD volume by a turbomolecular pump were conducted. Then
the body of the AD experimental cell was cooled down to the
studied temperature (the temperature of hydrogen saturation)
and spectrally pure hydrogen was injected with samples to a
given pressure in the volume of AD. Further, pressure change
in the AD volume with studied samples was recorded under the
preset saturation temperature using a deformation pressure
sensor. After that, the heating of ampoule device with samples
was stopped, and the samples were cooled in the hydrogen
atmosphere to room temperature. After 12 hours, samples were
heated again to a preset saturation temperature and kept under
this temperature shelf for 15-20 minutes, after which samples
were cooled to room temperature, and remaining hydrogen was
pumped from the volume of the ampoule device.
Research Results and Discussion
The main criteria that determine the prospects of application
of those or other materials for storing hydrogen, typically consider
the amount of their sorption capacity, operating temperature
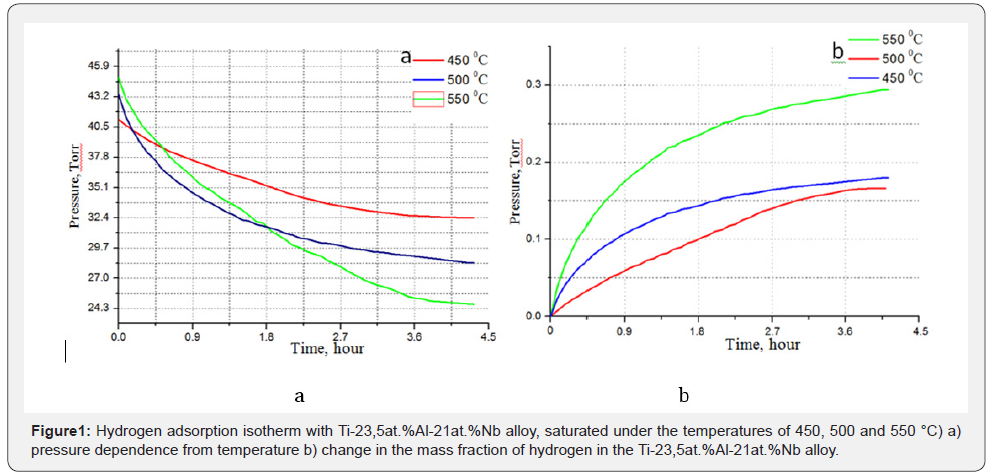
and pressure, kinetics of the interaction [8]. Сurves of hydrogen
sorption by Ti-23.5at.%Al-21at%Nb alloy under temperatures
of 450, 500 and 550°C and a pressure of 41 Torr (Figure 1) were
presented to compare processes of sorption isotherms. Figure
shows that under the increase of temperature from 450°C to
550 °C, an increase in the rate of hydrogen sorption occurs and
respectively, the change in pressure of the ampoule (Figure 1a)
is observed. Figure 1b shows the mass fraction of hydrogen
absorbed by the sorbent at temperatures of 450-550 °C.Figure
1b shows that there is an intensive absorption of hydrogen at a
temperature of 550 °C, the proportion of hydrogen absorption
reaches up to 0.289 mass.%. Probably, the interaction of Ti2AlNb
phases with hydrogen occurs firstly, traces of which are present
in the samples, thus the activation barrier of the reaction of the
material main phases is decreased.
The ampoule device was annealed at a temperature of 900
°C for 30 minutes, before the experiment of desorption with an
empty ampoule device. Argon was injected to one atmosphere
after walls of the ampoule device cooled to a temperature of
20 °C in the volume of the ampoule device, then the ampoule
device was closed, and the ampoule volume was pumped to a
pressure of 10-4Torr, after which the ampoule device was tested
for tightness using RGA-100 quadrupole mass spectrometer and
the helium. Further, the desorption process of hydrogen by Ti-
23.5at%Al-21at%Nb alloy was conducted. The heating was from
20 to 790 °C.
In the result of conducted experiments, the dependence
of the hydrogen pressure from the sample temperature at an
increase up to 790 °C (Figure 2) was obtained. Results of the
study of Ti-23.5at%Al-21at%Nb alloy desorption showed that
hydrogen release was observed in the temperature range of 700
... 790 °C. Maximum hydrogen content in the sample saturated
at 550 °C was 0.289 mass.%. Figure 2b shows that the hydrogen
release from a sample saturated at a temperature of 550 °C
reaches up to 85%.The active yield of hydrogen is observed at a
temperature of 750 °C.

The paper [9] presents the dependence of the desorption
pressure for some systems, which shows that hydrides based
on alloys of intermetallic compounds can be used to accumulate
hydrogen in a fairly wide range of temperatures and pressures.
The main factor limiting the rate of hydrogen release and
absorption by the accumulator, in most practically important
cases, is the heat and mass transfer in the layers of intermetallic
particles, and not the sorption-desorption kinetics on individual
particles [10].
The results of the study of hydrogen-adsorption properties
showed, that the pressure of hydrogen desorption increases
sharply at 500 °C. Thus, Ti-23.5at%Al-21at%Nb alloy is a high
temperature getter. Results of the study of hydrogen desorption
are presented in Table 1.
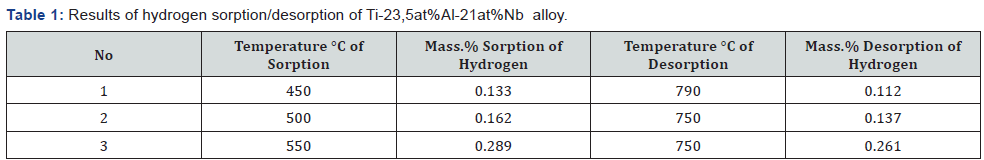
Thus, it was found that the rate of sorption/desorption
of hydrogen depends on the heating temperature. It is also
important to note that the orthorhombic phase of Ti2AlNb is a
well hydrogen absorber. This is confirmed by the absorption of
hydrogen at a sufficiently low pressure (about 45 Torr.), and can
be explained by the acceleration of diffusion in the Ti-Al system
by doped Nb.
Conclusion
Based on the analysis of the results, following conclusions
can be made:
a) The kinetics of hydrogen sorption by Ti-23.5at%Al-
21.5at%Nb at isothermal conditions under temperatures
of 450, 500 and 550 °C is studied. The dependence of the
mass fraction of hydrogen in the material samples from
temperature was obtained.It is determined that the maximum
amount of about 0.289 mass% of absorbed hydrogen is
observed in the material sorbed at a temperature of 550 °C;
b) It had been found that hydrogen is released under the
temperatures within 700 …790 °С. At the same time, the
chemical composition of the material samples practically does not affect the temperature modes of hydrogen release.
It was found that the maximum 85% of hydrogen release is
observed in saturated at a temperature of 550 °C.
For more
details Journal of Polymer Science please
click on: https://juniperpublishers.com/ajop/index.php
To read more…Full Text in Juniper Publishers click on https://juniperpublishers.com/ajop/AJOP.MS.ID.555562.php


Comments
Post a Comment