Recent Advances in the Synthesis and Analysis of Polyamide 6 and Products therefrom: From Polymerization Chemistry of εcaprolactam to Thermoplastic Resin Transfer Moulding (t-rtm)
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
Significant research and developments are taking place in the field of synthetic polyamides (Nylons). One of the most investigated members of this family of polymers is poly (ε-caprolactam) (PCL, Polyamide 6, PA6, Nylon 6). Herein, a brief overview will be presented on the major recent advancements in the anionic ringopening polymerization (AROP) of ε-caprolactam (CL) by the initiating combinations of metal lactamates and carbamoylcaprolactams and by some other initiating systems, e.g. protected N-heterocyclic carbenes , NaHMDI and σ-borane-barium complexes. The in situ polymerization of CL in the presence of additives, such as flame retardants, reinforcing agents and fillers, is also discussed. Examples are presented on the determination of the molecular weight distributions (MWD) and average molecular weights of Polyamide 6 by GPC (SEC) measurements with 1,1,1-trifluoroethanol as eluent used routinely in our laboratories. Finally, a glimpse will be provided into the ongoing intensive research related to thermoplastic resin transfer molding (T-RTM) processes aiming at producing Polyamide 6 products, such as reinforced PA6 and composites, by rapid in situ polymerization of CL.
Keywords:Polyamide 6; Nylon 6; ε-Caprolactam; Anionic ROP; Initiator; Activator; PA 6 additives; GPC (SEC); Thermoplastic Resin Transfer Moulding (T-RTM)
Introduction
Synthetic polyamides (Nylons) are still among the most important macromolecular materials with a wide variety of useful properties and applications even after nine decades of their discovery [1]. Significant research and developments are taking place with these polymers worldwide. These include exploring new polymerization chemistries for their production, modification of their structure and properties, and finding new ways of processing, especially by thermoplastic resin transfer molding (T-RTM), for a large variety of composites and products. In this brief overview, we attempt to provide a glimpse into the recent major advancement with one of the members of the Nylon family, Nylon 6 (Polyamide 6, PA6).
Polymerization of ε-Caprolactam: Effect of Catalysts, Additives, Fillers and Reinforcing Materials
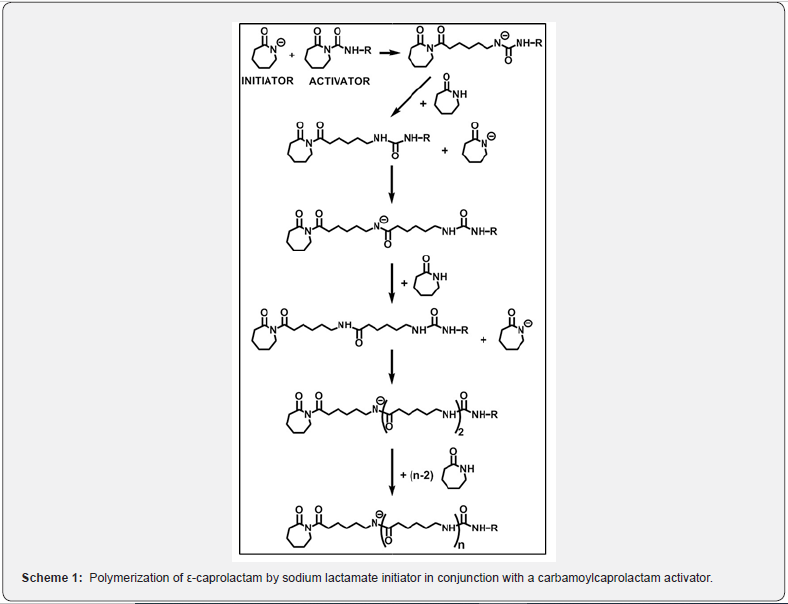
Beyond doubt, poly (ε-caprolactam) (i.e. Polyamide 6, PA6, Nylon 6) is one of the most widely investigated polymers due to its versatile properties and broad application possibilities. Polyamide 6 is mainly made by the anionic ring-opening polymerization (AROP) of ε-caprolactam (CL). The major current polymerization technologies are based on the AROP of CL by initiator-activator combinations [2-5]. The most widely used initiator-activator initiating systems include metal lactames (e.g. Na-, K-, Mg-lactames) in conjunction with carbamoylcaprolactams (CCL) such as hexamethylene-1,6-dicarbamoylcaprolactam. The major mechanistic steps of the polymerization of CL by such initiating systems are displayed in Scheme 1. One of the main advantages of this polymerization reaction is related to the fact that it can be carried out by bulk polymerization in the molten state of CL and the components of the initiating system, i.e. initiator and activator, to high yields without significant induction period at elevated temperatures. This solventless process with nearly quantitative monomer conversion without any side product can be considered as one of the most efficient green polymerization processes.
However, it is well-known that AROP is sensitive to moisture, which is able to inhibit the CL polymerization by reacting with either the components of the initiating systems or with the amide anion in the propagating chain [Scheme 1]. Interestingly, the effect of moisture on CL polymerization with industrial initiator and activator combinations has only recently been studied in a systematic manner by Wilhelm et al. [2,3]. They have found by polymerization kinetic investigations that the water present in the polymerization system of CL can be compensated by adding appropriate amounts of initiator and activator. Other systematic investigations on the effect of the condition of CL bulk polymerization with commercially used initiatoractivator mixtures are still lacking, although such studies are of paramount importance for the rapidly developing thermoplastic resin transfer molding (T-RTM) techniques, used for obtaining, in addition to other polymers, Polyamide 6 products as well. In one particular case, Kang and coworkers [4] have recently attempted to determine the optimal polymerization conditions for obtaining carbon fiber reinforced PA6 by TRTM with the use of lactamate-CCL initiating system by varying the injection rate of the components.
Investigations with other than lactamatecarbamoylcaprolactame initiating systmes have also been reported for AROP of CL. Buchmeiser et al. [6,7] carried out CL polymerization with latent, protected Nheterocyclic carbenes, and revealed the catalytic effect of such compounds as a function of the structure of the substituents. Sodium hydride (NaH) in combination with 4,4′-Methylenebis(phenyl isocyanate) (MDI) was used by Kim and coworkers [8] to initiate bulk (melt) CL polymerization to prepare Polyamide 6 composites with multiwalled carbon nanotubes. A complex of barium with σ-borane was successfully applied by Battacharjee et al. [9] to catalyze AROP of CL under mild conditions. Polymerization of CL in the presence of various additives is under intensive investigations nowadays. This can be considered as the major current trend in the field of the chemistry and processing of Polyamide 6, attempting mainly to reach short processing times to obtain advanced composite products, especially by TRTM. The following classes of additives have been recently investigated for the in-situ polymerization of CL by AROP: flame retardants [10-12], fillers, such as titania [13], zinc oxide [14], boron nitride [15] silica [16], montmorillonite [17] and multi-walled carbon nanotubes [8,18,19], and reinforcing agents, like glass fiber [20,21] and carbon fiber [4]. It has to be noted that in spite of its importance usually neither the CL monomer conversion nor the average molecular weights and the molecular weight distribution (MWD) are determined in such instances.
Characterization of Poly(ε-caprolactam) by Gel Permeation Chromatography
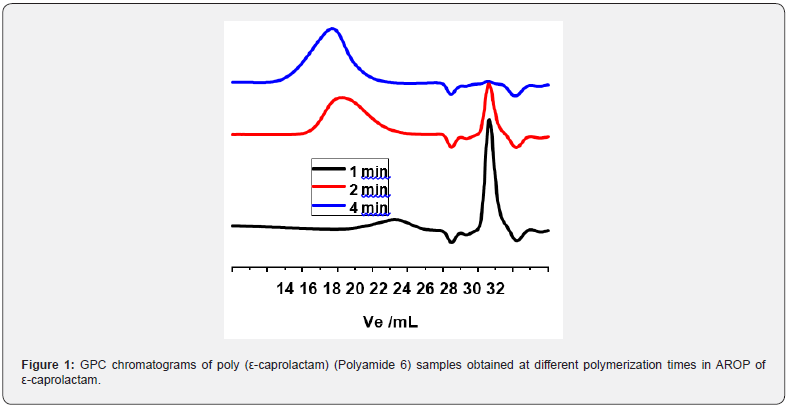
Although it is widely known that the MWD and the average molecular weights as well play critical role in the major properties of polymers for both processing and application purposes, it is quite surprising that MWD determinations of Polyamide 6 by GPC (called also size exclusion chromatography, SEC) is rather rare in the open literature. In the few recent instances, GPC of PA6 and CL copolymers has been carried out in solvents like hexafluoroisopropanol (HFIP) [22,23], DMF [24], THF (for low MW PA6) [19] and m-cresol (for CL copolymer) [25]. In the course of our recent investigations on CL polymerization, we have found that 1,1,1trifluorethanol (TFE), as an alternative to HFIP, can also be used effectively as mobile phase in GPC for the determination of the MWD and average MWs of PA6 with a broad range of molecular weights. As displayed in Figure 1, GPC can also be utilized to follow the progress of CL polymerization. In this Figure, the low MW peak, i.e. the peak at higher elution volumes, corresponds to the CL monomer. The gradual disappearance of the monomer peak and simultaneous increase of the polymer peak can definitely be applied to follow the polymerization process in addition to the determination of the MWD and the average MWs of the resulting Polyamide 6. These findings by us indicate that GPC with 1,1,1-trifluoroethanol as eluent can be routinely utilized for not only the determination of the MWD of Polyamide 6 products, but it can also be used to investigate the progress of the polymerization reaction of CL as well.
Polymerization of ε-Caprolactam by Thermoplastic Resin Transfer Moulding (T-RTM) for Obtaining Polyamide 6 and Its Composites
Intensive research and development is taking place worldwide with significant interest and activities in the field of T-RTM for producing the broadest possible range of products based on Polyamide 6 [2,4,21,26]. This is due to the fact that the melt (bulk) polymerization of CL, having low melt viscosity at elevated temperatures, can be carried out in situ in the presence of suitable initiator-activator combinations in a short time. Such a process provides economic and energy saving technological advantages over classical injection molding technologies which preferentially use preformed polymers. T-RTM processing of Polyamide 6 composites has been recently used with a variety of additives, such as reinforcing materials [4,18,19] and fillers [15-17]. However, investigations on the effect of the reaction conditions on the CL conversion and MWD of the resulting Polyamide 6 obtained under T-RTM or similar techniques are very rare. As mentioned earlier, Wendel et al. [2,3] explored the effect of water on the rate and outcome of CL polymerization in a T-RTM process. The optimization of the injection speed in T-RTM of CL by varying the sodium caprolactamate/CCL ratios in the presence of carbon fiber was recently attempted by Kang and coworkers [4]. However, systematic investigations on the effect of the major reaction parameters on the CL bulk (melt) polymerization with industrial reagents is still lacking. Considering this situation, our recent research efforts aim at revealing these correlations, which might be broadly utilized in T-RTM and other in situ bulk polymerization reactions with CL for finding optimal processing conditions [27].
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/


Comments
Post a Comment