Excited Triplet States of Organic Molecules and Reactive Free Radicals in Polymers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
The mini-review is devoted to the recent investigation the role of cage effect during photopolymerization. Benzophenone was selected as a photoinitiator of polymerization.
Introduction
Free-radical photopolymerization (UV-cure) of organic coatings is a very important process that has been used for more than half a century. Academic and industrial researchers study the basics of this complex process [1-4] In this mini review we will briefly consider the important role of low MW free radicals of photoinitiators (PIs) and the excited triplet states of PIs in photopolymerization. On the contrary, residual PIs in the cured coatings and other low MW photoreactive species in the cured coatings/polymers lead to a negative effect: photodecomposition of polymers outdoors, poor weatherability. We will comment on that as well.
Discussion
A primary chemical act of free radical photopolymerization is the generation of radical pairs (RP) in the triplet spin state. PI absorbs UV- or visible light; populates the excited singlet state of PI which in turn undergoes intersystem crossing into the triplet state. PIs often contain a carbonyl group in their structure. Reactive triplet state either undergoes dissociation into two radicals (Norrish 1 process) or H-abstraction from co-initiator or even from the coatings material or a polymer, both possessing C-H bonds. The first PIs are dubbed as Type 1 PIs, and the latter PIs as Type 2 PIs [4-6]. We will use a commercial PI benzophenone (BP) as an exemplar. Photochemistry of BP and reactions of corresponding free radicals BPH• have been extensively studied. Thus, the action of BP as a PI or as a residual PI in the cured coatings can be presented by eq. (1):
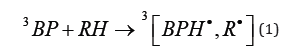
Here RH is a hydrogen donor. The RP formed in reaction (1) either decays in the polymer cage or radicals escape into the polymer bulk, as per (Scheme 1) shown below. The formation of an RP in the triplet state is a hurdle for a cage reaction. RP can react only in the singlet state with few exceptions. We include in our discussion low MW radicals which are formed at a high degree of photopolymerization (conversion of vinyl groups), say at . At high the media can be regarded as a polymer. A fraction of photogenerated radicals which decay inside a cage is called cage effect. Obviously, chemists are interested in the lowest possible in the case of photoinitiation of polymerization and in the highest possible in the polymers outdoors. (For fairness sake, we will mention that an aromatic radical BPH• does not initiate polymerization, but a counter radical R• does.) 3BP and R• are damaging species in the cured coatings or polymers that facilitate photodegradation. Photopolymerization usually does not lead to complete consumption of PI. On average 20% of the dissolved PI stays in the cured coatings. Being outdoors, the cured coating is subjected to destruction initiated in particular by the residual PI and possibly by carbonyl compounds formed during autoxidation. (Here we talk about processes which last years.) Thus, cage effect value and its dependence upon the degree of photopolymerization and properties of a polymer are very important for practice [7,8] It was demonstrated that the value of depends in particular upon the free volume of a polymer Vf and the glass transition temperature of a polymer Tg [3,9]. Cage effect dynamics (Scheme 1) since the inception of RP at t 0 till relatively long time upon completion (milliseconds) of cage reactions has been studied by ns flash photolysis with optical or ESR detection. This research was done mainly with PI=BP. 10 ns resolution of such instruments is okay. Cage reactions in the viscous media (polymers) lasts microseconds [1-3,9,10]. Application of moderate external magnetic field affects [1-3,7] and should find a practical application in the cure of coatings. An interesting type of coatings are coatings which can be photopolymerized without a PI [10,11].
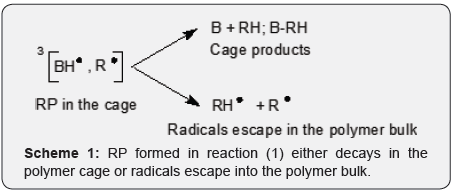
Conclusion
It is not surprising, that commercial PIs [4-6] are molecules that have a high quantum yield of an excited triplet state and which dissociate from a triplet state with a formation of a triplet RP [10]. Triplet RP has a high probability of dissociation and initiation of polymerization even in a viscous media.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/


Comments
Post a Comment