Preparation and Properties of Polyacrylamide/Titanium Oxide Hydrogels
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
In this study, using acrylamide (AAm) as monomer, potassium persulfate (K2S2O8) as initiator, N, N, N’, N’-tetramethylethylenediamine as accelerator, and N, N’-methylenebisacrylamide as crosslinking agents, titanium dioxide (TiO2) as inorganic enforcement fillers, the PAAm/TiO2 organic-inorganic composite hydrogels with enhanced mechanical properties were prepared by free radical polymerization. The obtained hydrogels were characterized by Fourier transform infrared spectroscopy (FT-IR), mechanical and swelling property tests. The experimental results show that PAAm/TiO2 organic-inorganic composite hydrogels were successfully synthesized, the mechanical property of the hydrogel was improved but the swelling ratio of the hydrogel was decreased due to the addition of TiO2.
Keywords: Acrylamide; Titanium Dioxide; Mechanical Property; Swelling; Composite Hydrogel
Introduction
Hydrogel is a new functional polymer material with three-dimensional network structure, which has been extensively studied due to its excellent properties including high water content, fast expansion, good biocompatibility, and good response to external stimulation. According to the type of the external stimuli, polymer hydrogels can be divided into temperature [1-3], pH [4-5], light [6-8], magnetic [9-10], pressure [11-12], electric [13] sensitive polymer hydrogels, which have been widely used in the areas such as biomedical materials, sensing, adsorption separation, and robotics [14-17]. The development of polymer hydrogels with functional or new properties has been a hot research topic. However, the current research and development of hydrogel materials is still not effectively to meet the increasing demands in complex devices and biomedical fields, mainly in the following areas: the mechanical strength of traditional hydrogel materials is relatively poor [18], and still different from the hydrogel in natural tissues; the application of hydrogel materials needs to be further expanded. In this study an organic-inorganic composite hydrogel material with improved mechanical properties was prepares using free radical copolymerization, and the mechanical and swelling properties of hydrogels were investigated. This study provides a method for improving the mechanical property of the hydrogel materials.
Materials and Methods
Chemicals and Materials
Acrylamide (AAm, 98%) and Titanium Dioxide (TiO2, 40-60 nm) of analytical grade were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China). polyethylene glycol (PEG, molecular weight 1500) of analytical grade was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). N, N, N’, N’-tetramethylethylenediamine (TMEDA, 98%), N, N’- Methylene bisacrylamide (MBA, 98%), and potassium persulfate (K2S2O8, 99%) were purchased from Shanghai J&K Chemical, Shanghai, China.
Synthesis of hydrogels
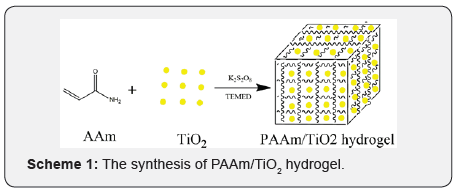
The scheme of hydrogel synthesis in this experiment is as follows: (Scheme 1)
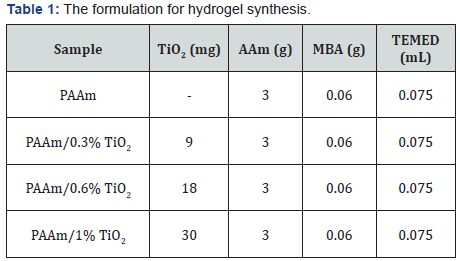
1.2 g of PEG and 0.72 g of K2S2O8 were dissolved in 60 mL and 30 mL of deionized water using the ultrasonic oscillator, respectively. The chemicals and reagents were scaled according to the formulation for hydrogel synthesis shown below (Table 1). A certain amount of TiO2 was mixed with 5 mL of PEG solution, and the mixture was placed in the ultrasonic oscillator for 10 min. Then 3 g of AAm and 0.06 g of MBA were added to the TiO2/PEG mixture, and treated in ultrasonic oscillator for 15 min. 2 mL of K2S2O8 solution was introduced, the whole dispersion was submerged in ultrasonic oscillator for 2 min and oxygen was removed by bubbling nitrogen through the mixture for 5 min.. Finally, 0.075 mL of TEMED (promoter) was added to the mixture and the reaction was allowed to take place at room temperature for 24 h. After the reaction, the hydrogel samples were purified in deionized water and small molecules diffused out. Every 8 h the deionized water was refreshed for two days. By changing the loading content of TiO2, the composite hydrogels with different mechanical property could be achieved.
Instruments and Characterization
Fourier transform infrared spectra (FT-IR) of samples in the form of KBr pellets were performed using a NICOLET IS 10 FTIR spectrometer at the range of 4000-400 cm-1. the compression tests were carried out at room temperature using a universal testing machine (WDT-1000, Shenzhen Kai Li Test Instrument Co., China) at a compression speed of 5 mm/min. The cylindrical samples of the as-prepared hydrogels were used, with dimensions of 20 mm in diameter and 25 mm in height. Swelling performance test was performed as follows: a certain amount of synthetic hydrogel sample was placed into a vacuum oven and dried to constant weight, and the mass of the dry hydrogel (m0) was accurately measured. Then the dry hydrogel was submerged into the deionized water. Every a few minutes the hydrogel was taken out from water and its surface was in contact with a dry and clean filter paper. The mass of the wet hydrogel (mt) was immediately recorded. After a certain period of swelling, the swelling ratio (SR) of the hydrogels can be calculated with the formula as follows:
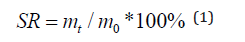
Results and Discussion
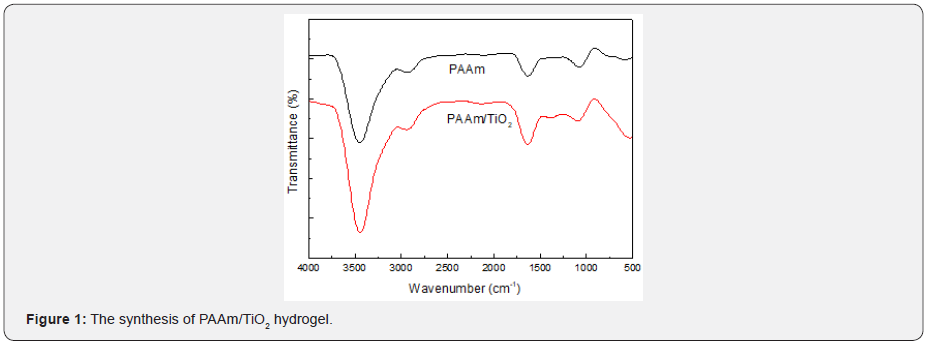
(Figure 1) shows the FT-IR spectra of Pure PAAm and PAAm/ TiO2 composite hydrogels. The content of TiO2 in PAAm/TiO2 composite hydrogels is 1%. As can be seen from (Figure 1), the spectrum of PAAm and PAAm/TiO2 composite hydrogels shows a broad band at 3460 cm-1, which is assigned to the stretching vibration of O-H and N-H from AAm units. The absorption peaks observed at 2930 cm-1 and 1630 cm-1 were corresponding to the characteristic stretching vibration of C-H and C=O from the AAm main chain. The result indicated that PAAm hydrogels was successfully synthesized. As long as TiO2 was evenly dispersed in a pure hydrogel, the PAAm/TiO2 composite hydrogel could be obtained. (Figure 2) shows pictures of pure PAAm and PAAm/TiO2 hydrogels before and after compression test. The content of TiO2 in PAAm/TiO2 composite hydrogels is 1%. It can be seen that the AAm hydrogel is colorless and transparent, while the hydrogel loaded with the inorganic particle TiO2 is white.

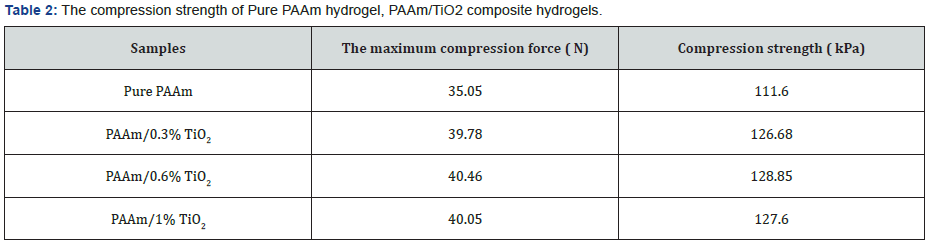
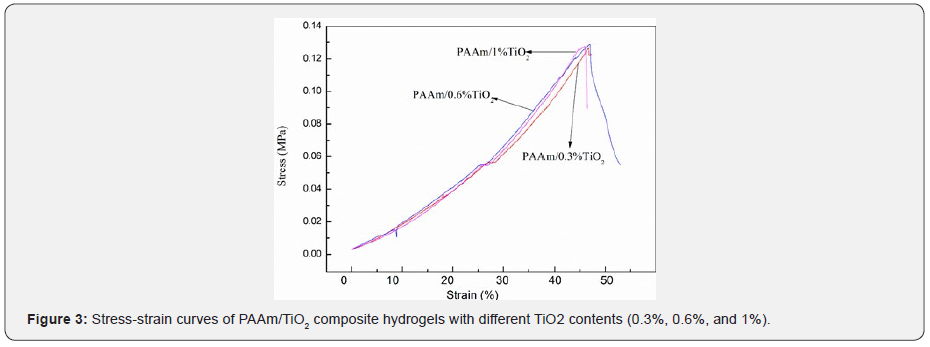
Hydrogel is deformed under the pressure of the universal tester, and the stress is equal to the ratio of the force provided to the contact surface area of the hydrogel, thus calculating the maximum stress of the specimen. (Table 2) and (Figure 3) show the mechanical properties data of pure hydrogels and composite hydrogels. It can be seen the maximum compression forces for pure PAAm hydrogels and PAAm/TiO2 composite hydrogels with different TiO2 contents were 35.05, 39.78, 40.46 and 40.05 N, respectively, which are corresponding to the compression strengths of 111.6, 126.68, 128.85, and 127.60 KPa, respectively. Strength refers to a material’s ability to resist the destruction (deformation and fracture) of external forces. As can be seen from (Figure 3), under the same stress, the PAAm/ TiO2 composite hydrogel has a smaller degree of deformation than the PAAm hydrogel. The PAAm/0.6% TiO2 composite hydrogel has the least degree of deformation and the highest strength. The PAAm hydrogel has the greatest degree of deformation and minimal strength. It can be seen that the addition of an appropriate amount of TiO2 improves the mechanical properties of the hydrogel. Toughness refers to the ability of a material to absorb energy before breaking. It can be seen from (Figure 3) that the strains of PAAm hydrogel and PAAm/ TiO2 composite hydrogels with different TiO2 contents (0.3%, 0.6%, 1%) are 48.16%, 47.36%, 46.97%, 45.82%, respectively. It can be seen that too much addition of TiO2 leads to a decrease in the brittleness and toughness of the hydrogel. Therefore, an appropriate amount of TiO2 can ensure good toughness of the PAAm/ TiO2 composite hydrogel. In summary, the proper addition of TiO2can improve the strength of the hydrogel.
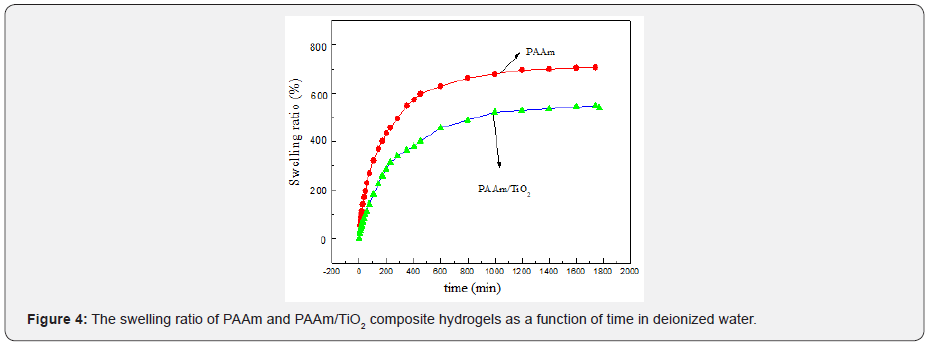
(Figure 4) displayed the swelling ratio of PAAm and PAAm/ TiO2 composite hydrogels as a function of time in deionized water at 25 oC. TiO2content in PAAm/ TiO2composite hydrogels was 1%. From (Figure 4), it can be seen that the expansion rate of hydrogel increases rapidly with time from 0 to 400 min, and after 400 min, the rate of swelliing gradually slows down until the swelling equilibrium is reached. The equilibrium swelling ratio of pure PAAm and PAAm/ TiO2 hydrogels were 707% and 539%, respectively. The balance swelling ratio of the composite hydrogel loaded with TiO2 is significantly smaller than that of pure hydrogel, mainly because of the presence of TiO2 inorganic particles, which can increase the additional physical crosslinking point, resulting in an increase in the crosslinking degree of hydrogels and a decrease in the degree of swelling.


Comments
Post a Comment