Synthesis and Radical Copolymerization of Novel Phenyl-Disubstituted Propyl Cyanoacrylates- Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
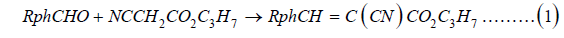
Abstract
Novel phenyl-disubstituted propyl 3-(R-phenyl)-2-cyanoacrylates, RPhCH=C(CN)CO2C3H7
(where R is 2-fluoro-5-methyl, 3-iodo-4-methoxy, 5-iodo-2-methoxy,
3,5-dichloro, 3,4-difluoro, 3,5-difluoro, 2-chloro-4-fluoro,
2-chloro-6-fluoro, 3-chloro-2-fluoro, and 3-chloro-4-fluoro) were
prepared using condensation of substituted benzoic aldehydes and propyl
ester of cyanoacetic acid. The ethynyl benzene copolymerization of novel
cyanoacrylates was conducted in solution with radical initiation at 70
C. Nitrogen analysis, 1H & 13C NMR was used to analyze composition and the structure. Thermal behavior of the copolymers was analyzed by DSC and TGA.
Keywords: Radical copolymerization; Styrene copolymers; Trisubstituted ethylene’s; Cyanoacrylates
Introduction
Ring–functionalized trisubstituted ethylene’s (TSE), esters of 3-phenyl-2-cyanoacrylates, R1PhCH=C(CN)
CO2R2 continue to attract attention as compounds
with interesting properties and as comonomers for modification of
commercial polymers. 3,4-Difluorophenyl substituted methyl
3-phenyl-2-cyanoacrylate was used in synthesis and studies of histamine
H2 agonistic activity [1]. Ethyl 3-phenyl-2-cyanoacrylate was used in
studies of stereoselective cascade assembling of
(1R*,2S*)-1-cyano-5,7-dialkyl-4,6,8-trioxo-2-aryl-5,7-diazaspiro[2.5]octane-1-carboxylates
[2] and analgesic activity of 6-fluoroindan-1-carboxylic acid [3].
Alkyl 2-cyanoacrylates are a family of vinyl monomers renowned for their
high reactivity, instant adhesive properties, and wide-ranging
applications [4].
We have reported synthesis and ethynylbenzene
copolymerization of similar ring-substituted methyl [5], ethyl [6],
& butyl 3-phenyl-2-cyanoacrylates [7]. With the objective to design
novel structures, that could serve as a spring board for further
development of novel materials with new properties and applications, we
have prepared halogen ring-disubstituted propyl 3-phenyl-2-cyanoacrylate
(PPCA), RPhCH=C(CN)CO2C3H7sub>,
where R is 2-fluoro-5-methyl, 3-iodo-4-methoxy, 5-iodo-2-methoxy,
3,5-dichloro, 3,4-difluoro, 3,5-difluoro, 2-chloro-4-fluoro,
2-chloro-6-fluoro, 3-chloro-2-fluoro, and 3-chloro-4-fluoro,
and copolymerize them with ethynylbenzene. To the best of our knowledge,
there have been no reports on either synthesis of these propyl phenyl
cyanoacrylates, nor their copolymerization with ethynylbenzene.
Experimental
All benzoic aldehydes, propyl cyanoacetate,
piperidine, ethynylbenzene, 1,1’-azobiscyclohexanecarbonitrile, (ABCN),
and toluene supplied from Sigma-Aldrich Co., were used as received. The
melting points and Tg, were measured with TA Model Q10 DSC. The thermal
stability of the copolymers was measured by TGA TA Model Q50 from
ambient temperature to 800 ºC at 20 ºC/min. The MW of the copolymers was
determined by GPC using an Altech 426 HPLC pump at an elution rate of
1.0mL/min; Phenogel 5μ Linear column at 25 ºC and Viscotek 302 detector.
1H & 13C NMR spectra were obtained on 10-25% (w/v) monomer or polymer solutions in CDCl3sub>
at ambient temperature using Avance 300MHz spectrometer. Elemental
analyses were performed by Midwest Microlab, LLC (IN).
Results and Discussion
PPCA synthesis
The ring-substituted propyl 3-phenyl-2-cyanoacrylates
(PPCA) were synthesized by Knoevenagel condensation [8] of a
ring-substituted benzoic aldehyde with propyl cyanoacetate,
catalyzed by base, piperidine (1).
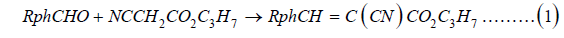
Where R is 2-fluoro-5-methyl, 3-iodo-4-methoxy, 5-iodo-
2-methoxy, 3,5-dichloro, 3,4-difluoro, 3,5-difluoro, 2-chloro-
4-fluoro, 2-chloro-6-fluoro, 3-chloro-2-fluoro, & 3-chloro-4-
fluoro. The preparation procedure was essentially the same for
all the monomers. In a typical synthesis, equimolar amounts
of propyl cyanoacetate and an appropriate ring-substituted
benzoic aldehyde were mixed in equimolar ratio in a 20mL
vial. A few drops of piperidine were added with stirring. The
product of the reaction was isolated by filtration and purified
by crystallization from 2-propanol. The condensation reaction
proceeded smoothly, yielding products, which were purified by
conventional techniques.
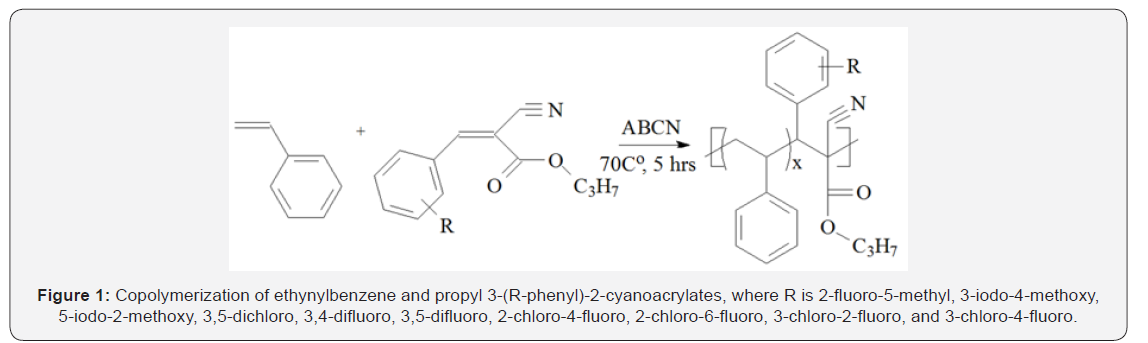
Copolymerization
The PPCA monomers when mixed with ethynylbenzene,
EB at EB/PPCA = 3 (mol) formed copolymers as indicated by
white flaky precipitates in high excess of methyl alcohol. The
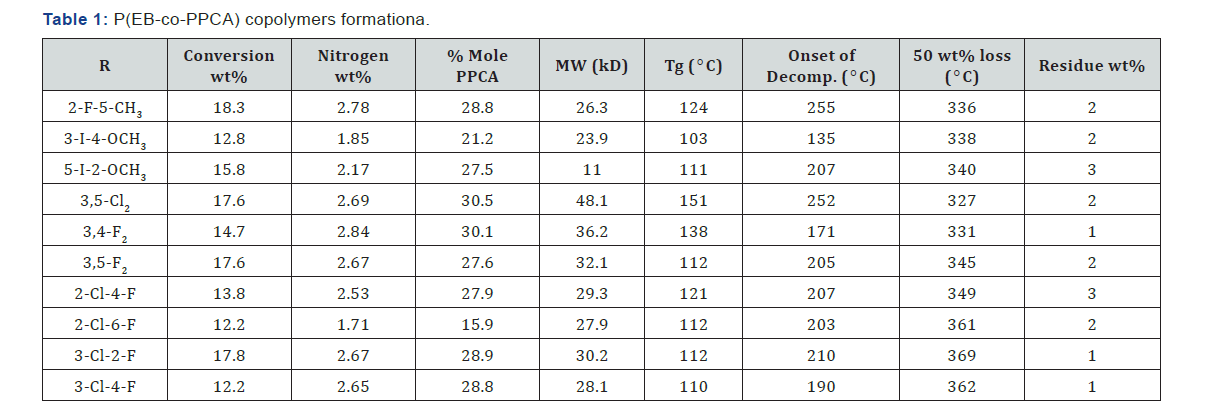
conversion of the EB-PPCA copolymers was maintained 10 - 20%
to decrease compositional drift (Table 1). The PPCA content 8.4-
25.8 mol% in the copolymers indicated relatively high reactivity
of the PPCA monomers towards EB radical Figure 1.


The EB-PPCA copolymers were soluble in tetrahydrofuran,
ethyl acetate, chloroform and dimethylformamide, and insoluble
in methyl alcohol, ethyl and petroleum ethers. GPC analysis the
copolymers indicated weight-average molecular masses 10.7-
27.8kD (Table 1). According to elemental analysis, between 18.3
& 42.6 mol% of PPCA monomer is present in the copolymers
prepared at EB/PPCA = 3 (mol), which is indicative of relatively
high reactivity of the monomers towards EB. Results of DSC and
TGA analyses are presented in Table 1.
The FTIR spectra of the monomers were compared with
those of copolymers and poly(ethynylbenzene) thus providing
evidence that the reaction between the PPCA monomers and
EB is a copolymerization. The spectra of the copolymers show
overlapping bands in 3200-2820cm-1 region corresponding to
C-H stretch vibrations. The bands for the PPCA monomer unit
are 2246-2238 (w, CN), 1752-1733 (s, C=O), and 1252-1226cm-1
(m, C-O). Benzene rings of both monomers show ring stretching
bands at 1500-1400cm-1 as well as a doublet 824-715cm-1,
associated with C-H out of plane deformations. These bands can
be readily identified in ethynylbenzene copolymers with TSE
monomers containing cyano and carbonyl electron withdrawing
groups.
EB-PPCA copolymers microstructure analysis is based on
1H and 13C NMR spectroscopy (DEPT, HETCOR, NOESY and
JMODXH) of EB copolymers with 2-phenyl-1,1-dicyanoethene
[9] which showed the formation of both head-to-tail and headto-
head alternating monomer structures, as well as short EB
sequences.
Conclusion
Novel phenyl-disubstituted propyl 2-cyano-3-phenyl-2-propenoates
were prepared and copolymerized with ethynylbenzene.
The compositions of the copolymers were calculated from
nitrogen analysis and the structures were analyzed by IR, H1 &
13C-NMR. The thermal gravimetric analysis indicated that the
copolymers decompose in in two steps, first in the 200-500C
range with residue (1-3%wt), which then decomposed in the
500-800 ºC range.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com


Comments
Post a Comment