Polymerization of 4-Vinyl-1-Cyclohexene Diepoxide by Rhenium Carbonyls Compounds- Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
The polymerization of 4-vinyl-1-cyclohexene diepoxide
(4-VCHD) by rhenium carbonyl, Re(CO)5X (X = Br, Cl) and dirhenium
decacarbonyls Re2(CO)10 has been carried out
photochemically at 25oC, and thermally at 25 and 75oC witout
cocatalysts. The effects of initiator structure, concentrations, and
reaction temperature and time on the polymerization rate is reported
here.
Keywords: 4-vinyl-1-cyclohexene diepoxide; Rhenium Carbonyl; Photoinitiated Polymeriation
Introduction
Diepoxy resins have found important commercial
applications in UV radiation curing of surface coatings; a dhesives and
in the plastic industry [1,2]. Photoinitiated cationic ring-opening
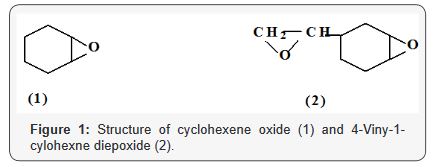
polymerizations of cyclohexene oxide (CHO)1 (Figure 1) was conducted
using dirhenium decacarbonyl [3] and rhenium carbonyl halides [4]
without a cocatalyst. 4-vinyl-1-cyclohexene diepoxide 2 is used as a
reactive diluent’s for diepoxides and epoxy resins; this monomer is
expected to form crosslinked polymers, if both epoxide groups are
polymerized. Cationic photoolymerization of 4-VCHD by diphenyliodonium
hexafluorophosphate was carried out and a mixture of crosslinked and
benzene soluble polymers were obtained [5], cationic thermal
polymerization by BF3. O(C2H5)2 [6], and it has been reported that two
epoxy rings can be opened for polymerization selectively by radiation,
but not by chemical initiators.
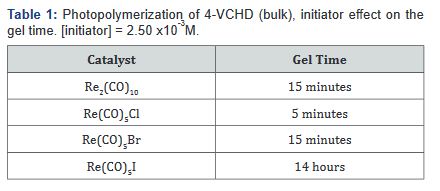
In this paper we report on the polymerization of
4-VCHD (Table 1) by rhenium carbonyl, Re(CO)5X (X = Br, Cl) and
dirhenium decacarbonyls Re2(CO)10 photochemically and thermally without
cocatalyst Table 2. Insoluble polymer was obtained in photo or thermal
polymerization even at low conversion of monomer.
Experimental
Materials
4-vinyl-1-cyclohexene diepoxide (Fluka) was distilled
over calcium hydride (CaH2), and the middle fraction was collected.
Solvent dichloromethane (Fluka) were dried over calcium hydride and
distilled before use. Rhenium carbonyls were obtained from Pressure
Chemical Company and used as received.
Instruments
Ultraviolet spectra were obtained on a Cary 2300
spectrophotometer. Infrared spectra were recorded on a Nicolet 50xB
FT-IR spectrophotometer.
Polymerization
Photoinitiated polymerization was carried out in a
15mm diameter Pyrex tube using a tight syringe for monomer addition; a
homogeneous solution was formed, the reaction tube was then closed with
rubber septum, and irradiation was carried out using a merry-go-round
photoreactor, Model RPR 100, which rotates continuously by a motor and
is surrounded by 16 Hanovia 450
Watt, medium pressure mercury. The light source was equipped
with 350 nm wavelength tubes. The samples were placed in the
holder and irradiated for the required period.Thermal polymerization
was conducted by placing the reaction tube in a water bath
at the required temperature for the time indicated in dark. Isolated
polymer was washed with dichloroethane, filtered, dried and
weighed. The rate of polymerization was calculated gravimetrically
as a function of reaction time [7].
Results and Discussion
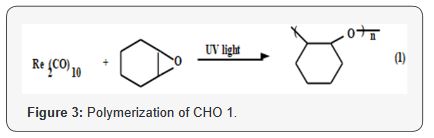
Polymerization of CHO monomer 1 proceeds through the
opening of the epoxide ring to give soluble polymer poly (cyclohexene
oxide), the product is long sequences of cyclohexane rings
interlinked by oxygen atoms, Figure 3, however; the polymerization
of the diepoxide monomer 2 was found to proceeds through
the opening of both epoxide ring to give crosslinked polymer of
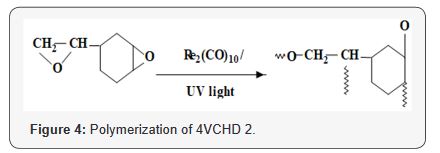
poly (4-VCHD), as shown in Figure 4. Evidence for the structure
of poly 4VCHD was obtained by studying the FTIR spectrum of
the polymer obtained under different conditions. Typical epoxide
bands characteristic of the monomer at 890, 850 and 913cm-1
are missing from the polymer spectra, and a new very powerful
band at 1087 and 1157cm-1 associated with the ether linkage is
present. The new band at 108 cm-1 is the strongest, and its position
varies slightly with chemical structure of the polymer. The
important characteristic in the polymerization reaction of 4-VCHD
by rhenium carbonyls is the start of the polymerization of both of
the epoxy rings at the early stages of polymerization, a crosslinked
polymer was obtained at 2% conversion is an idication of reaction
of both epoxides at the same time (Figure 4). Thermal polymerization
of 4VCHD by dirhenium decacarbonyl and the rhenium
pentacarbonyl halides is shown in (Table 2).
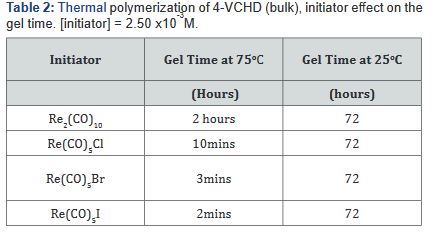
Re(CO)5Cl > Re(CO)5Br = Re2(CO)10 >> Re(CO)5I
Thermal polymerization of monomer 2 by the rhenium carbonyl
halides proceed very slowly at 25oC, and the gel time is 72
hours; and for polymerization at 75oC the gel time fall in the following
sequence: Re(CO)5I > Re2(CO)10 > Re(CO)5Br > Re(CO)5Cl
(Table 2). This activity is in acrodance with the bond strength between
the halide and the rhenium atom. Polymer obtained as a
white powder, insoluble in all solvents.
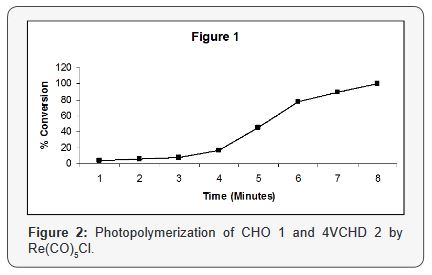
A comparison beteen the bulk photopolymerization of CHO
1 and 4VCHD 2 under the same conditions using Re(CO)5Cl
(2.50x10-3M), shows the required time for complete polymerization
(100% conversion) of CHO 1 is 10 minutes and for 4VCHD 2 is
5 minutes, this indicates that the reactivity of monomer 2 almost
is twice as that of monomr 1, and both epoxide rings react and
opened at the same time.
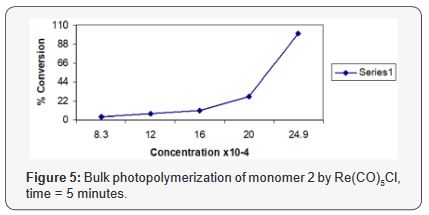
Effect of initiator concentration
The effect of Re(CO)5Cl concentration on 4VCHD photopolymerization
in the range (1.80 x10-3 to 3.69 x10-3M) for fxied time
of 5 minutes and without solvent is shown in Figure 5, this indicates
an increase in the rate of polymerization as the initiator concentration
increases, polymer obtained as crystalline solid which
is insoluble in aromatic and halogenated hydrocarbon solvents.
Poly4-VCHD characterization
The polymer structure was identified by FTIR spectroscopy.
The FT-IR spectrum of poly (4-VCHD) prepared photochemically
and thermally by Re(CO)5Cl and Re2(CO)10. Poly (4-VCHD) prepared
photochemically by by Re2(CO)10 shows the OH group at
3400, aliphatic (CH2, CH) at 2960, 2850, and 1920, 1720 (CO) and
1655, 1470 ( six member ring in polymer), 1440, 1050, 1087 and
913cm-1 for the C-O-C stretching frequency [8,9]. These assignment
suggest that the catalyst is attached to the poly (4-VCHD).
Proposed polymerization mechanism
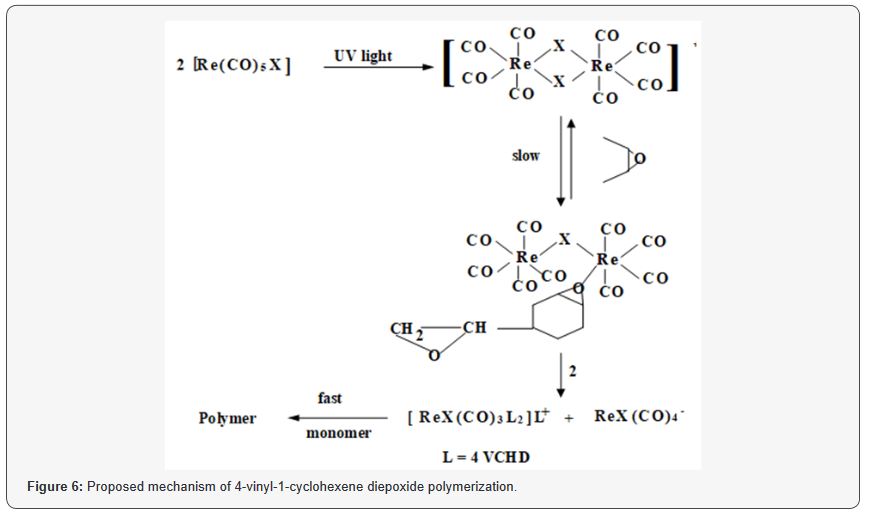
The photodisproportionation of the complex (Re (CO)5Cl is
shown in (Figure 6), L represent a coordinating monomer. The
dimerization of the photoexited Re(CO)5X is bridged through the
halogen (X), the bridges is broken by the monomer (4-VCHD), further
addition of the monomer to the complex induce the initial
propagation reaction. For photoinitiation of the polymerization of
4-VCHD by Re2(CO)10 compounds, we suggested the same mechanism
as reported for photopolymerization of cyclohexene oxide
[8], the growth of the two epoxide functional groups will leads to
crosslinked polymer(Figure 6).
Conclusion
Rhenium carbonyls are effective photoinitiator for the
polymerization of (4-VCHD) without cocatalyst, in absence of UV
light long reaction time is required. Polymerization by Rhenium
carbonyls shows that both epoxide ring were opened. The rate
of polymerization depends on the structure of the rhenium
carbonyl. Insoluble polymer was obtained in photo or thermal
polymerization even at low conversion of monomer.
Acknowledgement
Support to this work from university of sharjah is greatfully
acknowledged.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com


Comments
Post a Comment