Formulation and Characterization of Glutathione-Loaded Bioadhesive Hydrogel for Ocular Delivery-Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
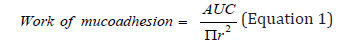
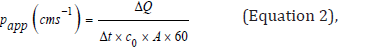
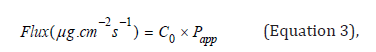


Abstract
Glutathione may prevent age-related, oxidative damage
to ocular tissues but has poor corneal penetration. Hydrogel
formulations were investigated to determine an optimized ocular delivery
system. The rheology and texture profile of formulations were
investigated at 25°C. The 1% w/v glutathione was incorporated into
systems demonstrating desirable characteristics for ocular vehicles and
physical characteristics re-determined. In vitro cumulative glutathione
delivery across the bovine cornea was measured over 8 hours at 32ºC in
Franz diffusion cells. Carbopol-containing Poloxamer systems exhibited
shear-thinning behavior desirable for ocular formulations whilst
Polyvinyl alcohol (PVA) and Polyacrylic acid (PAA) systems exhibited
Newtonian behavior. Of the glutathione-containing systems, 0.2% w/v
Carbopol 1342 was the most viscous with a viscosity of 960cP at a shear
rate of 100sec-1. All formulations significantly increased the amount of
glutathione delivered across the cornea (relative to an aqueous
solution of glutathione) with the exception of 0.1% w/v Carbopol 940
(p=0.12). Formulations containing 0.1% w/v Carbopol 934, 0.1% w/v
Carbopol 1342 and 0.2% w/v Carbopol 940 improved penetration
dramatically (ca. 30%); but were not significantly different from each
other. Therefore, Carbopol and Poloxamer formulations demonstrated
enhanced penetration of glutathione across the cornea. The 0.2% w/v
C940-containing-Poloxamer formulation was determined to be the most
promising for ocular delivery of glutathione.
Keywords: Glutathione; Hydrogel; Ocular delivery; Rheology; Newtonian behavior; Sustained release; Permeation
Introduction
Glutathione (γ-glutamyl-cysteinyl-glycine; GSH) is a
highly hydrophilic thiol tri-peptide with a low-molecular weight of
307.4Da [1,2] The reduced form of GSH serves as a strong intracellular
antioxidant [1,3] and plays an important role in the protection of the
eye from oxidative stress [4]. In addition to its antioxidant activity,
glutathione serves as a cofactor of enzymes involved in the degradation
of peroxides, such as glutathione peroxidises. Moreover, as a component
of NADPH pathway, it prevents cell components from being oxidized thus
promoting DNA regulation and protein synthesis [3]. Franco et al [5]
also reported glutathione’s role in the regulation of cell-cycle, signal
transduction and immune response [5]. Given that all ocular tissues
contain GSH as a primary antioxidant, replenishing the amounts of GSH in
the eye could reduce the incidence of ocular diseases related to
oxidative stress and aging, such as cataracts. The cornea is
particularly susceptible to chemical insults and damage because it is
the outer-most layer of the eye and therefore exposed to the harsh
environment. GSH maintains normal hydration, protects the cellular
membrane and degrades xenobiotic agents [4] in the cornea. Therefore,
GSH may play a role in the treatment of keratitis and other corneal
diseases [4].
Due to anatomical and physiological characteristics,
the eye is uniquely shielded and a highly protected organ that presents
many barriers to effective ocular drug delivery [6]. The main physical
barriers shielding the eye are tear film and cornea, which protect the
anterior of the eye, Tear film is a protective layer that prevents the
entry of foreign molecules into the eye. The blood-retina barrier, which
protects the back of the eye [7]. The cornea is the main barrier
against drug penetration to the inner tissues of the eye due to its
small surface area and relative impermeability [8]. In addition, many
ocular enzymes in the cornea could degrade glutathione and prevent its
therapeutic effect. These enzymes include endopeptidases (plasmin,
collagenase) and exopeptidases, such as the hydrolytic aminopeptidase,
which degrade amino acids [9].
The main advantages of stopical administration are
its ease and convenience of use and localized drug effects, thus,
reducing systemic absorption and avoiding enzymatic degradation through
hepatic first pass metabolism [10]. However, the natural ocular defense
mechanisms lead to poor bioavailability. Blinking increases the
production of the tear film’s aqueous layer which in turn increases tear
turnover to approximately 1μL/min, which is equivalent to 16% of the
tear film turnover [11]. Both the removal
of excess fluid and tear turnover reduce the extent of drug absorption.
Conventional eye drops require frequent administration,
with each drop being more than 30μl, which is estimated to be
the maximum volume the eye can accommodate without overflow.
Drainage of instilled solution occurs within 15-30 seconds after
administration [12], therefore the majority of the drugs administered
are removed before absorption. Although ocular ointments
have the viscosity to increase pre-ocular drug retention time, they
often cause irritability to the eye, resulting in lower compliance.
In addition, their oily medium is not compatible with water soluble
drugs such as GSH. Consequently, an ocular formulation with
desirable characteristics that shows promising potential would
be required to effectively deliver glutathione. This delivery system
should prevent enzymatic degradation of glutathione, enhance its
pre-corneal retention and penetration.
Hydrogels can be formed by dispersing polymers in an aqueous
medium where they undergo swelling to produce a viscous
gel capable of increasing pre-corneal retention time, prolonging
contact time by increasing mucoadhesion and controlling drug
release [12]. These properties increase trans-corneal absorption
and hence the amount of drug reaching the anterior chamber of
the eye. Poloxamers are non-ionic triblock copolymers comprised
of ethylene oxide and propylene oxide, which are non-irritating to
the ocular surface. With favorable characteristics such as strong
hydrogen-bonding, high MW and sufficient flexibility to interact
with the mucus network contribute to excellent mucoadhesion.
Another defining characteristic include their ability to increased
stability of drugs, Carbopol (CP) is a synthetic high-MW polymer
comprised of acrylic acid that are cross-linked with either allyl
ether of pentaerythritol or allyl sucrose [3]. They are anionic and
therefore offer increased mucoadhesion hence contact time [13].
In addition, CP is reported to open the cellular tight junctions and
could promote trans-corneal penetration of glutathione [14]. PVA
has been used extensively in ocular formulations due to its ability
to maintain ocular osmotic pressure while PAA has been widely
used as a viscosity enhancer [2]. In this project, we developed a
novel hydrogel formulation which composed of CP, PAA and PVA,
and used as a carrier system for ocular delivery of GSH. This delivery
system is aimed to enhance the permeation of the GSH over
the cornea, thus, to maximize the bioavailability of GSH via ocular
administration.
Materials and Methods
L-glutathione reduced≥99% was purchased from Sigma-Aldrich
(USA). P407 and P188 were purchased from BASF (Germany).
C934NF were from Noveon (USA) while C940NF and
C1342NF polymer were purchased from Lubrizol (USA). PVA
came from Applichem (Germany) and PAA was from Polyscience
Inc (USA). Sodium hydroxide (NaOH) was from Scharlau (Spain).
All other chemicals and solvents were of analytical grade. Bovine
eyes were obtained from Auckland Meat Processors (New Zealand)
and stored at20°C until required.
Methods
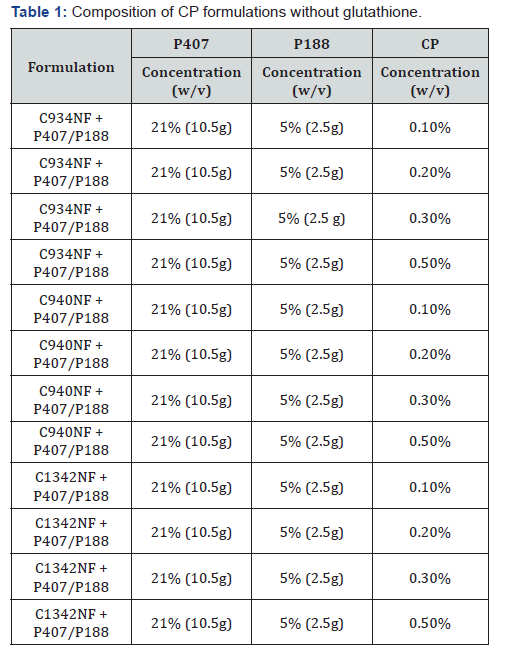
Preparation of Formulations
The CP formulations were prepared by adding the required
amount of CP to 35mL of milli-Q water and were continuously
stirred until the CP was completely dissolved. The solution was
cooled to 4°C and the required amount of Poloxamer 407 (P407)
and Poloxamer 188 (P188) were added with gentle stirring. The
formulation was stored in the refrigerator and stirred every 30
minutes until the Poloxamers were completely dissolved. Milli-Q
water was added to make up to a final volume of 50mL. The pH
of the formulations was then adjusted to 7.4 by the addition of a
small amount of 1M of NaOH. (Table 1) lists the samples prepared
using the method mentioned above.


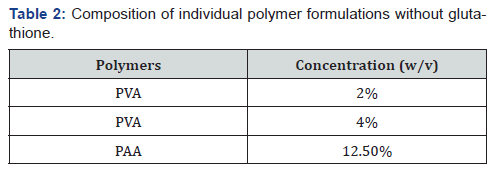
PVA formulations (Table 2) were prepared by adding the required
amount (w/v) to 35mL of milli-Q water, which was then
heated to 50°C under constant stirring until all PVA was dissolved
and made up to 50mL with milli-Q water. The pH was adjusted to 7.4 by adding a small amount of 5M NaOH. The corresponding
concentration of PAA samples were added according to (Table 2)
and dilutions were made with milli-Q water. The control used in
the screening of the formulations was milli-Q water.

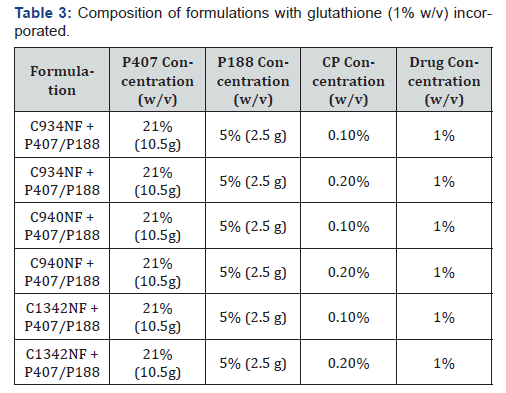
The polymer combinations were shown in (Table 3). The CP
formulations containing glutathione were prepared by dissolving
0.5g of glutathione in milli-Q water. Once glutathione was dissolved,
the required quantity of CP was added. The solutions were
continuously stirred until CP was completely dispersed and the
Poloxamers were prepared according to the method described
above. The control for samples mentioned in (Table 3) was aqueous
solution of glutathione. Simulated tear fluid (STF) was prepared
based on the method used by Hagerstrom et al [15]. Based
on the physical parameters obtained, several potential formulations
with glutathione will be investigated in this study.
Characterization
Rheological Properties
The rheological properties were examined using the Brookfield
DV-III+ rheometer (Brookfield Engineering Laboratories,
Inc. USA) with spindle 40 for PVA and PAA samples and spindle
52 for the CP formulations. This was carried out in a temperature-
controlled environment at 25±1°C to simulate average room
conditions. Each sample went through 20-40 loops of shearing, in
between which there was a 10 second delay. The speed of shearing
increased by 5rpm at each loop and the torque was kept between
9 to 90% by manipulating the starting rpm to ensure accuracy of
data. The range of rotational speed was between 1 and 394 rpm
corresponding to a shear rate range between 2 and 788s-1.
Mechanical and Mucoadhesive Properties
TA-XT plus texture analyzer (Stable Micro Systems, UK) with a
5kg load cell at 25±1°C was used. Texture analyzer was calibrated.
For the measurement of the mechanical properties, a 10mm diameter
delrin cylinder probe was twice compressed into each formulation
at the rate of 2 mms-1 to a depth of 5mm with a trigger force
of 0.1g. A delay of 15 seconds was allowed between the compressions.
Data collection and calculations were gathered from the
Texture Exponent 3.0.5.0. The force-time plot measured mechanical
properties such as hardness, compressibility, adhesiveness
and cohesiveness. This experiment was conducted in triplicate.
Hardness is measured by the peak force of the first compression
cycle. Compressibility is measured as the positive force area
during the first compression of the probe [3,16]. Adhesiveness is
the negative force area of the first compression [16]. Cohesive
force of each sample is the ratio of the first and second positive
force area of the two consecutive compressions.
Mucoadhesive force measurements were performed on freshly
prepared bovine cornea using TA-XTplus texture analyzer (Stable
Micro Systems, UK) with a 5kg load cell at 25±1°C. The cornea
was stabilised on the mucoadhesion test rig. A small amount of
sample was applied to the 10mm delrin cylinder probe surface,
after which the probe was lowered at a constant speed of 2mms-1
and a trigger force of 5g. After 10 seconds of contact, the probe
was moved away at a speed of 0.5mms-1, generating a curve. The
area under the curve (AUC) was calculated by the Texture Exponent
3.0.5.0 program. The work of mucoadhesion was calculated
through Equation 1[3]. Each measurement was conducted in triplicates.
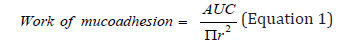
where πr² is the surface area of the cornea.
Permeability Studies
Ex-vivo drug permeation studies were performed by Franz
diffusion cell (VTC 200, Logan Instrument Corp). The thermostat
of the Franz diffusion cells was set at 32±1°C to mimic the ocular
surface temperature. Bovine cornea is placed between the donor
and the receptor chamber. Twelve mL of STF served as the dissolution
medium in the receptor chamber which functioned as the
reservoir. One mL of formulation was placed in the donor chamber
and the cap was covered with parafilm to prevent evaporation.
Samples (0.4mL) were collected at predeterminate time intervals
and replaced by 0.4mL of STF for the first four hours, after which
a greater amount of sample was withdrawn and replaced in order
to maintain sink condition. UV spectrometer was blanked
with STF at the wavelength of 215 nm and the absorbance of each
sample was obtained. The absorbance for samples presented in
Table 3 was measured and concentrations were calculated. In order
to evaluate the penetration rate, the apparent permeability
coefficient (Papp) was calculated, using the following equations:
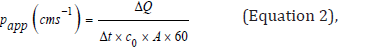
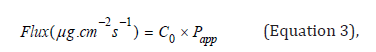
where ΔQ / Δt is the steady-state of the linear
portion of the
graph which presents the amount of drug in the receptor chamber
versus time, A represents corneal area available for diffusion
(1.766cm2), Co is the initial glutathione concentration in the donor
chamber and 60 is the conversion factor from minute to second
[2,17]. The linear branch of the permeation data was determined
using correlation analysis. A minimum of six data points in the linear
branch were taken to calculate the flux, J, (μg.cm-2s-1) by linear
regression. The flux was then divided by the concentration in the
donor (μg.cm-3) in order to calculate Papp (cm.s-1) [2,18].
Cryo-Scanning Electron Microscopy (Cryo-SEM)
Cryo-SEM was used to evaluate the hydrogel structure at
swollen state. Images were obtained through Philips XL30S FEG
(Field Emission Gun) SEM (Netherlands). Cryo unit, Gattan Alto
2500 was employed, including a fracture stage and a sputter
coater, with a coating temperature of less than -120ºC. Samples
were placed on a brass specimen holder and heated to allow thermogellation.
The sample were frozen by using liquid nitrogen
(-200ºC) and then cut and allowed to heat to -90ºC under vacuum.
This allowed the frozen water in between gel pores to evaporate,
generating the clear structure of polymer hydrogel. The surface
of the sample was sputtered with gold for 4 minutes at -120ºC,
to minimize any charge builds up, after which the samples were
viewed under the cryo-SEM.
Statistical Analysis
Statistical data was analyzed using Microsoft Excel 2007, with
two-way variance analysis (two-way ANOVA) and pair wise comparisons
were performed using t-tests. p<0.05 indicates a significant
difference.
Results
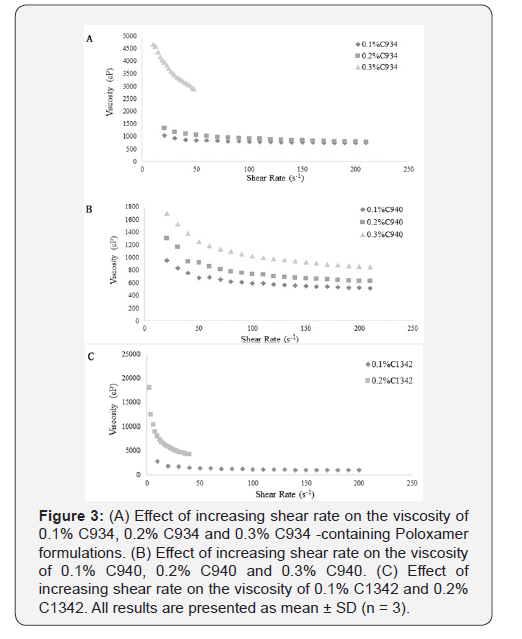
Characterization of Rheological Properties
Hydrogel ocular formulations should ideally have a viscosity
of around 1000-5000 cP in order to maximize pre-ocular retention
time and the delivery of glutathione. Formulations with
similar viscosities were plotted together for clear and apparent
analysis. The 0.3% C1342 formulation was not included as it was
too viscous and the method that was used to evaluate rheological
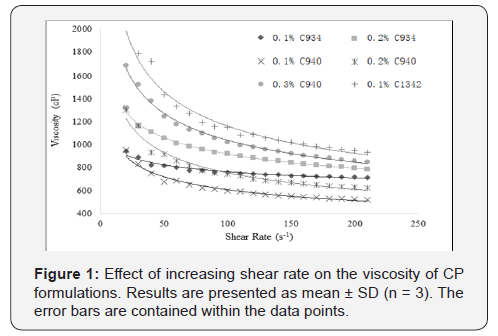
properties was not suitable [19]. All CP dispersions exhibited
non-Newtonian shear-thinning (pseudoplastic) behavior (Figure
1) i.e. decreasing viscosity with increasing shear rate.

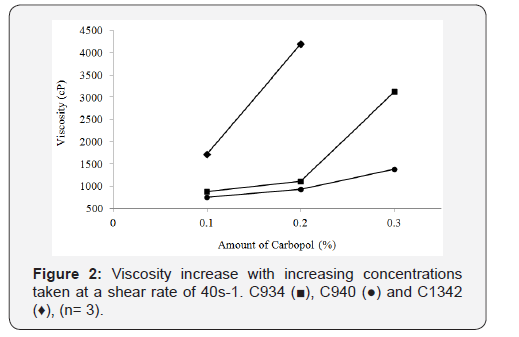
It was observed that the increase in viscosity with increasing
CP concentration was proportionally similar at 0.1% and 0.2% for
C934 and C940 at a shear rate of 40s-1. Conversely, C934 exhibited
a much greater increase in viscosity at 0.3% compared to that of
C940 (Figure 2). The viscosity difference appears to be proportional
between 0.1% and 0.2% of C1342 and 0.2% and 0.3% C934
at the shear rate of 40s-1. Moreover, the 0.1% and 0.2% C940-containing
formulations were the least viscous, while the respective
C934 formulations appeared to be three-times more viscous than
C940-containing formulations (Figure 2). The difference in viscosity
between all formulations at different concentrations appears
to be significant apart from that between 0.1% C934 and
0.2% C940 and between 0.1% C1342 and 0.3% C940.



The difference in viscosity at different concentrations for each
CP was statistically significant (p<0.05) (Figure 3A, 3B & 3C). The
C940 exhibited the most proportional increase in viscosity with
increasing concentration (Figure 3B); while the viscosities of
C934 and C1342 appeared to have been more dramatically affected
by the change in concentration. The rheological characteristics
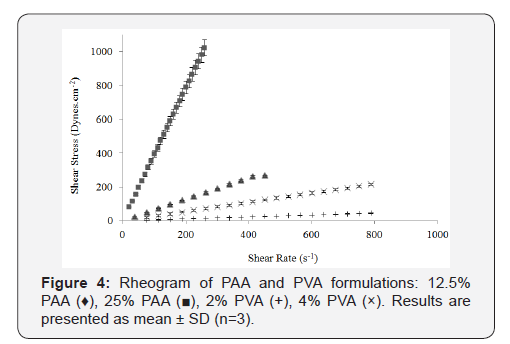
of PVA and PAA formulations are shown in (Figure 4). PVA and
PAA results were analyzed separately from that of the CP formulations
because their viscosity range was vastly different. The PVA
and PAA formulations demonstrated Newtonian flow, where a
linear relationship between shear rate and shear stress was evident
(Figure 4) [7]. Furthermore, the Newtonian flow properties
showed that the viscosity of these formulations remained constant
despite increasing shear rate. The 25% PAA is approximately
eight-times more viscous than the diluted 12.5% PAA and has
a much higher shear stress than all the other three formulations.
Similar to the CP systems, the viscosity of PAA and PVA also increased
with increasing concentration. Again, there was a significant
difference between the viscosities of these simple chain polymers
where PAA appears to be more viscous than PVA. The 25%
PAA displays a viscosity which is comparable to 0.1% C940-Poloxamer
formulation after shearing (Figure 2).
Based on the optimal ocular formulation characteristic described
by existing literatures available, the formulation demonstrating
the most desirable rheological properties contains the
0.2% C940, it exhibited the lowest viscosity at the lowest shear
rate investigated.
Characterization of mechanical and mucoadhesive properties
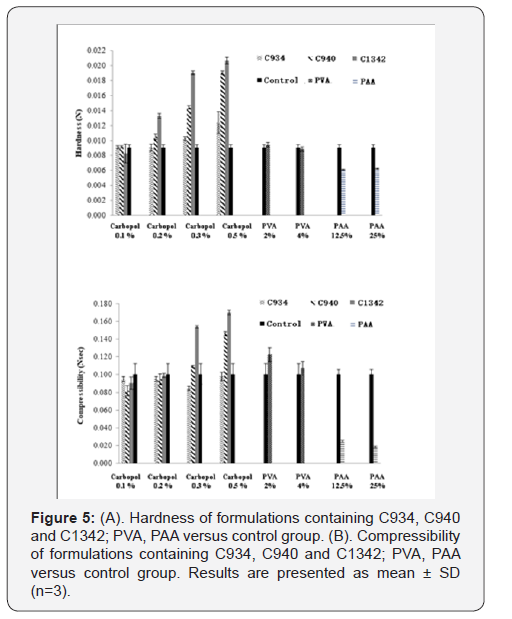
The hardness and compressibility of the hydrogel system
during formulation screening increased with an increase in CP
concentration. This trend can be observed for all CP-containing
formulations (Figure 5A & 5B). Significant increase in hardness
for C940 and C1342-containing systems was observed at concentration
of 0.2% compared to concentration of 0.1% and there was
significant difference compared to control (p<0.05). However, no
significant differences between the CP systems and control at 0.1%
(p>0.05) were observed. At 0.3% and 0.5%, all CP systems were
significantly different, compared to control (p < 0.05). Conversely,
PVA showed no distinctive change compared to control and no
significant differences between the different concentrations was
observed (p>0.05). PAA exhibited a significantly lower hardness
compared to control (p<0.05), however, there was no difference
when concentration was doubled. The hardness of the hydrogel in
the presence of PVA was no different when it was compared to all
0.1% CP formulations (p>0.05). The results obtained were comparable
to the previous literature data [20]. The compressibility
of the hydrogel in the presence of C1342 at 0.3% and 0.5%, as well
as C940 0.5%, was significantly different when compared against
control (p<0.05). Similar to hardness, PAA showed a significantly
lower compressibility in comparison to control (p<0.05).

A relationship exists between the increase in adhesion and
a decrease in cohesion of formulations (Figure 6A & 6B), with a
concentration increase. At 0.1%, C1342-containing formulations
were significantly higher compared to control (p<0.05). However,
adhesion of all CP-containing formulations increased with an
increase in concentration. Conversely, for PVA and PAA formulation,
no such trend can be observed with increase in concentration,
and statistical analysis showed no significant difference
when compared to control (p > 0.05). At 0.1%, 0.2% and 0.3% of
C934 and C94 containing systems, the cohesive forces were significantly
higher when compared to control (p<0.05), but this
was not demonstrated at a concentration of 0.5% (p>0.05). Cohesion of 0.1% and 0.2% of C1342-containing formulations were
significantly higher to control (p < 0.05). The cohesion forces of
0.1%, 0.2% and 0.3% of C934-containing formulations were significantly
different compared to 0.5% of C1342, C940 and 0.1%
of C940 respectively (p<0.05). The PVA and PAA formulations
demonstrated higher cohesive forces than control (p<0.05) and
showed a concentration dependent relationship similar to the CP
formulations. There was no significant difference in cohesion between
PVA and control (p>0.05), but both PAA concentrations had
significantly lower cohesion compared to control (p<0.05).


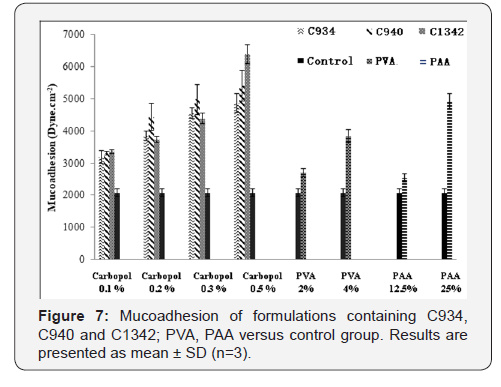
The measured apparent mucoadhesion showed a difference
between the control and all formulations screened (Figure 7). It
also showed a consistent increase in mucoadhesive force with
increase in concentration, however, there was no significant difference
between different CPs at concentrations 0.1% and 0.3%.
At 0.2%, only apparent mucoadhesion of C940 was significantly
different to that of other CPs. At 0.5% all CP formulations were
significantly different from each other. PVA and PAA showed a
similar trend, PAA at 25% obtained similar results to 0.5% C934
containing formulation.
Corneal permeability studies



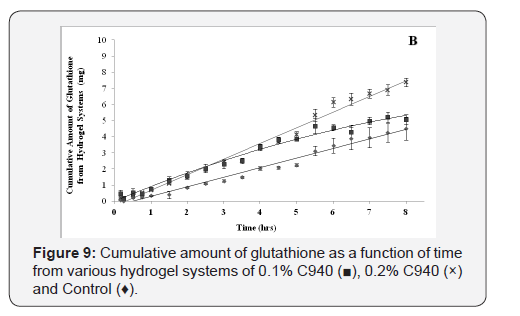
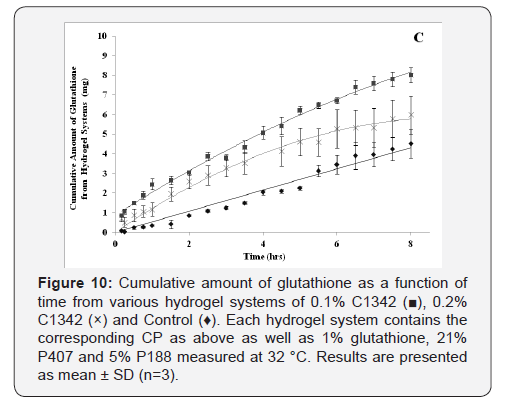
The cumulative diffusion profiles of each various hydrogel formulations
of glutathione over eight hours are shown in (Figure
8-10). All of the hydrogel systems showed a linear diffusion trend
and showed a significantly higher diffusion when compared to the
control (p<0.05). The permeation of 0.1% C940 formulation was
significantly less than 0.1% C934, 0.1% and 0.2% C1342 (p<0.05).
The remaining formulations did not show any differences when
compared to each other. The ranking order of the different formulation
groups in their ability to promote glutathione diffusion
across bovine cornea is: 0.2% C940 and 0.1% C1342 > 0.1% C934,
0.2% C1342 and 0.2% C934 > 0.1% C940 > Control.
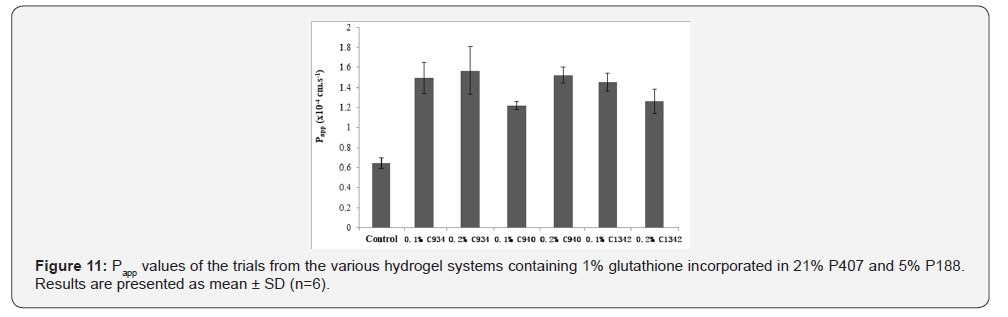
The apparent permeability coefficient (Papp) and Flux values
were calculated and shown in (Figure 11). From the Papp of the
different formulations measured, the permeability of glutathione
was increased compared to water (p<0.01). The 0.2% C940
(p=0.03) and 0.1% C1342 (p=0.05) had an approximately 1.2-fold
higher corneal permeability than 0.1% C940. All other hydrogel
formulations did not show differences in their permeability (p >
0.05). The average Papp values of the hydrogels were in the range
of 1.21-1.57 × 10-4 cm.s-1 compared to 0.64×10-4cm.s-1 produced
by the control. This shows that the CP combinations enhance the
permeability of drug by up to a 2.4-fold (p<0.05).


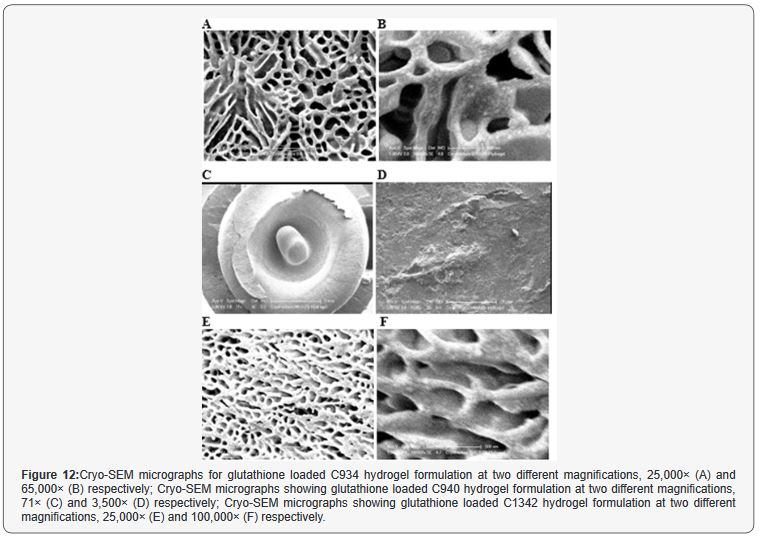
The Cryo-SEM images demonstrate the polymeric structure
of the achieved CP hydrogel systems. Images of C934 and C1342
hydrogel systems with incorporated glutathione were obtained
(Figure 12). Although, attempt was made to obtain images of C940
hydrogel structure, this could not be achieved due to experimental
error. An air bubble was formed during preparation and, as it
was cut, no formulation remained on the metallic rib for imaging;
thus, the images of C940 hydrogel structure were not obtained
and displayed.
Discussion
In order to increase the bioavailability of glutathione (and
hence achieve post-corneal concentration), the pre-corneal residence
time or penetration of the ocular formulation must be extended
beyond that of the conventional formulations [21]. This
may be achieved by increasing the viscosity, spreadability or mucoadhesion
of the formulations; or by incorporating permeation
enhancing materials into the formulation. The simplest approach
is to enhance the viscosity because a two-fold increase in maximum
bioavailability of a drug can be achieved. Therefore, viscosity
is often considered as the major factor influencing ocular retention
[12]. The increase in retention time of instilled formulations
is apparent, once viscosity is greater than 10mPas but a very high
viscosity results in ocular discomfort and epithelial damage can
occur from blinking [22]. The difference in the effective number
of polymers evaluated was likely the reason for the higher shear
stress observed in this experiment. Upon the addition of NaOH
solution to adjust pH to 7.4, carboxyl groups on the carbomers
(pKa 3-5) are ionized, leading to electrostatic repulsion and dramatically
increasing the rigidity of the formulation structure [23].
Thus, the ionized carboxylic groups could have also interacted
with the small but very positive sodium ions, leading to a reduction
in viscosity and shear stress. This theory could have been
validated had the rheological characterization been carried out at
both pH 5 and pH 7. Although Davies et al. [12] found the viscosity
profile of PVA to be pseudoplastic in contrast to our findings; the
conditions they used were extreme (85oC) and would not occur in
the eye. Moreover, Davies et al. [12] prepared their PVA solution
with PBS, introducing ions that possibly change the interactions
between the molecules. Nevertheless, C934 was found to have
greater viscosity-enhancing capability than PVA. Structurally, PAA
are CPs without cross-link units and can be described as linear
polymers (pKa~4.5) [24]. Although PAA has similar rigidity as
the carbomers, it is less viscous because the strength provided by
cross-links is much more robust.
The structures of hydrogels are highly dependent upon the
interaction between the polymers and the swelling medium [25].
The mechanical properties of the hydrogel formulations were assessed
through hardness, compressibility, adhesion and cohesion.
The ability to prolong ocular contact time was measured through
its strength of mucoadhesion [20,26,27]. The hardness is desired
to be low in order for the formulation to be easily administered
onto the ocular surface [27,28]. Therefore, lower concentrations
of CP formulations (0.1% and 0.2%) are favoured. The “hardness”
of anionic gels is dependent on the molecular network density
and the degree of cross-linking, which is correlated to the polymer
concentration, as well as viscosity [29-31]. C1342-containing
hydrogels exerted the highest “hardness” out of all three CPs. This
is likely to be due to increased cross-linking between its long alkyl
methacrylate chain and allyl ethers of pentaerythritol and its
high molecular weight compared to C934 and C940 [32]. Similarly,
C934 with relatively lower molecular weight was the easiest
formulation to deform due to its reduced rigidity. Additionally,
surface molecules have a net inward force due to the cohesive
forces being stronger than adhesive forces with the air, therefore
a surface energy is created in parallel to the surface. This requires
energy to break, which would have been measured as hardness
[33-35]. The hardness of PAA was lower than the control. This
may be explained through the accumulation of PAA molecules on
the surface, which would push the liquid molecules into the bulk,
leading to reduction in surface tension [33,34]. Compressibility
claimed to measure the ease of spread of the formulations over
the corneal surface; where a lower value was favorable. With increasing
concentration, total resistance was greater hence more
force was required as the parameter quantifies deformation under
shear and compression [28]. The claimed “cohesion” is described
as the spatial reformation of the hydrogel structure after
successive compressions. It attempted to measure the relaxation
of the polymers, which is dependent on time and the deformation
[36]. The “cohesion” of the formulation hoped to examine the ability
of the hydrogel to reform its structure after application to the
ocular surface, where a high value was desired for maintenance
of its structural integrity [27]. A general decline in cohesive force
is shown with increase in concentration, which is due to the increase
in mass of the polymers [37]. The adhesion is defined to be
the interaction between different molecules within the hydrogel.
This estimates the ability of the formulation to adhere to the target
site [20]. At higher concentrations, there were more polymers
present with greater capability to form chemical bonds [19]. The
results showed a plateau at concentrations 0.3% and 0.5% for all
CP-containing formulations. This may be explained by the high cohesive
forces within the hydrogel structure to prevent adhesive
forces forming with the probe [28]. The degree of mucoadhesion
is dependent on the hydration, anionic charges, molecular weight
and cross-linking of the polymer [32,38]. All CP-containing formulations
were combinations of Poloxamers and CPs. This combination
increased its mucoadhesive properties as the polyether components
of Poloxamers were able to form tertiary carbon bonds
with polyacrylic acid units of CPs, which may potentially expose
different binding groups to increase mucoadhesive interactions
[34,39]. Moreover, the CP-containing systems at 0.1% and 0.2%
had a greater degree of swelling, therefore increased hydration.
The polymers are flexible and free to diffuse to come in close contact
with the corneal surface and interpenetrate with the mucus
layer, to create a strong entangled network through non-covalent
interactions [19,38].
The cumulative amount of glutathione that diffused across
the cornea from the original administered dose (1mL), after eight
hours ranged from 50.6% to 80.1% from the hydrogels compared
to 45.0% from the control system. This is very high compared to
the 5% or less, which is typically seen to reach the aqueous humour
in living human eyes [40]. This is due to the absence of other
ocular barriers such as tear turnover, tear dilution, lacrimation
and nasal lacrimal drainage in the Franz diffusion cell apparatus
[40]. The glutathione control group follows a linear trend across
the corneal epithelial membrane and represents zero-order kinetics
where diffusion was at a constant rate throughout the experiment.
Conversely, the glutathione-loaded CP-containing hydrogels
show a biphasic release profile. The initial phase was attributable
to the release of glutathione from the outer pores of the gel surface
and the second phase showed the sustained release of glutathione
from the matrix of the polymer. This release profile shows
benefits on application, rapid diffusion of glutathione through the
corneal epithelial membrane will occur, followed by a sustained
diffusion over time.
Conclusion
Glutathione is a potent anti-oxidant that is essential in the
maintenance of tissue health. By replenishing the tissues with
glutathione, it is hoped that the progression of ocular damages
can be halted or even reversed. Hydrogels composed of CPPoloxamer,
PAA and PVA were formulated and investigated as
potential delivery systems for glutathione across the cornea. The
CP-Poloxamer combinations exhibited favourable pseudoplastic
behavior and cohesion/adhesion properties. These were further
investigated for their ability to increase glutathione permeability
across excised bovine corneas using a Franz diffusion cell.
Penetration of glutathione across the cornea was significantly
increased compared to the plain drug solution. The 0.2% C940-
containing-Poloxamer formulation was determined to be the most
promising for ocular delivery of glutathione. To study the efficacy
of glutathione formulations on the reversibility of cataracts, in
vivo tests can be performed in the future.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on:https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com


Comments
Post a Comment