Thermodynamics of Biomass-Based Solid Fuels-Juniper Publishers
JUNIPER
PUBLISHERS- ACADEMIC JOURNAL OF POLYMER SCIENCE
Abstract
In this paper, solid fuels made of plant biomass were
studied as an alternative to fossil coals. For this purpose,
experimental and calculation methods were applied to determine the
standard change of internal energy or specific energy of combustion
)(ociUΔ standard enthalpies of combustion ()ociHΔ and formation ()ofiHΔ
for individaual components of plant biomass (lignin, cellulose,
hemicelluloses, extractives, etc.), as well as of some additives of
solid biofuels. The experiments were carried out using an oxygen bomb
calorimeter, whereas calculations were performed by the equation:
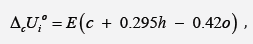
where E = -413 kJ/mol, c, h and o is number of atoms
C, H and O, respectively, in molecule of organic substance or in repeat
unit of polymer. Using the results obtained for individual components,
the standard thermodynamic characteristics (TDC), oY , of various
biomasses and their based fuels were found according to additivity rule,
as follows: ooiiYwY=Σ , where iw is weight part of the component in the
biofuel. The results revealed that calculated TDC the solid fuels were
close to experimentally obtained characteristics. The obtained data
evidence on adequacy of the additivity rule to evaluate the TDC of solid
biofuels. It has been also found that fuel pellets consisting of plant
biomass and additive of plastic binders are the most promising solid
fuels, since they provide a higher value of thermal energy and increased
energy density than the biomass only.
Keywords: Plant biomass, Biofuel; Cellulose; Hemicelluloses; Lignin; Extractives; Thermodynamic characteristics; Calorimetry; Calculation
Introduction
Currently, the main solid fuels are fossil coals,
which provides 28-30% of annual energy consumption in the world, about
160-180EJ [1]. To generate such energy, more than 6 billion tons of coal
are burned each year. However, this fossil source of energy is not
reproduced in nature, and therefore its reserves are permanently
depleted. Besides, the burning of coals is accompanied by emission of
greenhouse gas - carbon dioxide, in the huge volume of 1700-1800m3 from
each ton, which can exacerbate the problem of global warming [2].
An alternative to coal can be a solid fuel based on
plant biomass, which in contrast to this fossil fuel, is reproduced in
nature. The term “biomass” means here a variety of plant materials, as
well as their residues and wastes [3]. Diverse types of biomass can be
used to produce of solid fuels such as soft- and hardwood; herbaceous
plants (e.g. Miscanthus, Switchgrass, Bermuda grass, etc.); forest
residues (e.g. sawdust, twigs, shrubs, etc.); residues of agricultural
plants (e.g. stalks, husks, cobs, etc.); residues, waste and trash of
textile, pulp, paper, cardboard and plants, as well as algae biomass,
etc. Total amount of available biomass aimed especially for energy
production is estimated at 8-10 billion tons [4].
As is known, all constituents of biomass are
photosynthesized in chlorophyll pigment of plant leaves from carbon
dioxide and
water, absorbing red and blue-violet sunlight [5,6]. In fact, plant
biomass can be considered an accumulator of solar energy captured during
photosynthesis. To generate the heat energy, the biomass is burned,
resulting in the release of accumulated solar energy. A specific feature
of the biomass that it is neutral for emission of carbon dioxide, since
its combustion produces the same amount of this greenhouse gas as it
was absorbed from the atmosphere during photosynthesis.
However, the plant biomass is a heterogeneous
material having low bulk density, which consists of pieces of different
shapes, sizes and compositions. These negative features lead to
deterioration in fuel properties - unstable calorific value, low density
of thermal energy and insufficient combustion efficiency [7]. To
increase the low energetic density, the loose biomass should be
converted into dense pellets. Nevertheless, even after compaction, other
negative biomass characteristics remain, such as low calorific value
and sensitivity of the solid fuel to water absorption. A promising way
to improve the fuel features of biomass is the use of plastic binders in
the pelletization process [8]. As is known, large amounts of plastic
waste about 250-300 million tons are thrown out annually and pollute the
environment [9]. After separation of PET bottles from plastic debris,
the main fraction of the plastic waste consists of polyolefins, which
can be used as additive to biomass for co-firing.
The use of biomass and its mixtures with polymer binders
for production of solid fuels requires knowledge of standard
specific energy of combustion ( 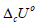 )
and other Thermodynamic
Characteristics (TDC). Numerous attempts have been performed
to determine the
)
and other Thermodynamic
Characteristics (TDC). Numerous attempts have been performed
to determine the 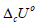 values for certain types of biomass and
synthetic polymers using the precise experimental method,
namely a combustion of sample in a bomb calorimeter [10-13],
as well as various calculation methods [13-16]. However, the
calorimetric measurements are lengthy and requires multiple
repetitions to obtain a reliable result; besides bomb calorimeter
is a complex, expensive and not always available device. On the
other hand, to calculate the
values for certain types of biomass and
synthetic polymers using the precise experimental method,
namely a combustion of sample in a bomb calorimeter [10-13],
as well as various calculation methods [13-16]. However, the
calorimetric measurements are lengthy and requires multiple
repetitions to obtain a reliable result; besides bomb calorimeter
is a complex, expensive and not always available device. On the
other hand, to calculate the 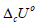 value for each biomass type, its
own equation has been proposed [13-15]. Since an unlimited
number of different types of biomass and its based compositions
exists, an unlimited number of experiments or calculations are
required to determine the TDC of various solid fuels, which is
impossible to realize.
value for each biomass type, its
own equation has been proposed [13-15]. Since an unlimited
number of different types of biomass and its based compositions
exists, an unlimited number of experiments or calculations are
required to determine the TDC of various solid fuels, which is
impossible to realize.
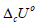 )
and other Thermodynamic
Characteristics (TDC). Numerous attempts have been performed
to determine the
)
and other Thermodynamic
Characteristics (TDC). Numerous attempts have been performed
to determine the 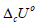 values for certain types of biomass and
synthetic polymers using the precise experimental method,
namely a combustion of sample in a bomb calorimeter [10-13],
as well as various calculation methods [13-16]. However, the
calorimetric measurements are lengthy and requires multiple
repetitions to obtain a reliable result; besides bomb calorimeter
is a complex, expensive and not always available device. On the
other hand, to calculate the
values for certain types of biomass and
synthetic polymers using the precise experimental method,
namely a combustion of sample in a bomb calorimeter [10-13],
as well as various calculation methods [13-16]. However, the
calorimetric measurements are lengthy and requires multiple
repetitions to obtain a reliable result; besides bomb calorimeter
is a complex, expensive and not always available device. On the
other hand, to calculate the 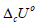 value for each biomass type, its
own equation has been proposed [13-15]. Since an unlimited
number of different types of biomass and its based compositions
exists, an unlimited number of experiments or calculations are
required to determine the TDC of various solid fuels, which is
impossible to realize.
value for each biomass type, its
own equation has been proposed [13-15]. Since an unlimited
number of different types of biomass and its based compositions
exists, an unlimited number of experiments or calculations are
required to determine the TDC of various solid fuels, which is
impossible to realize.
For this reason, another approach should be used. As is
known, any plant biomass contains cellulose, hemicelluloses,
lignin, extractives and small amounts of some other substances.
Besides, mixed solid fuel can contain also plastic additive. Thus,
to find the TDC of solid fuels made of biomass or its mixture with
plastics, it is sufficient to determine the TDC for limited number of
individual components and their content in the sample and then
use the additivity rule.
Although some TDC of individual components of plant biomass
has been studied by various researchers, the obtained results were
insufficiently reliable since they were varied over a wide range.
For example, a considerable variation in the 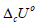 values for lignin
samples, from 20 to 29 kJ/g was observed [15,17,18], whereas
the variation in specific energy of combustion for cellulose and
hemicellulose samples was from 16.9 to 18.6 kJ/g [10,15,17-19].
values for lignin
samples, from 20 to 29 kJ/g was observed [15,17,18], whereas
the variation in specific energy of combustion for cellulose and
hemicellulose samples was from 16.9 to 18.6 kJ/g [10,15,17-19].
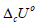 values for lignin
samples, from 20 to 29 kJ/g was observed [15,17,18], whereas
the variation in specific energy of combustion for cellulose and
hemicellulose samples was from 16.9 to 18.6 kJ/g [10,15,17-19].
values for lignin
samples, from 20 to 29 kJ/g was observed [15,17,18], whereas
the variation in specific energy of combustion for cellulose and
hemicellulose samples was from 16.9 to 18.6 kJ/g [10,15,17-19].
The purpose of this study was an accurate determination of
standard thermodynamic characteristics of individual components
of biomass, as well as of some plastic binders, in order to use the
obtained results to evaluate the TDC of solid biofuels by means of
additivity rule.
Experimental
Samples
Various types of biomass were used such as chips of spruce,
pine and poplar, corn, wheat straw, switchgrass and bagasse of
sugarcane, as well as waste paper and cardboard. The biomass
samples were cut, knife-milled and screened through a sieve to
obtain the fraction of 1-2mm.
The samples of bleached chemical grade Cotton Cellulose
(COC) and Kraft Pulp (KRP) were used. Besides cellulose was
isolated from Bagasse (BAC), Wheat Straw (WSC), Corn Stalks
(CSC) and Switchgrass (SGC) by a two-stage pulping using dilute
nitric acid and alkali with additional bleaching [20]. The cellulose
samples were additionally purified by extraction with organic
solvents, chelating agent (EDTA), boiling 2% NaOH and boiling
water to neutral pH; then samples were rinsed with deionized
water, ethanol and dried at 380 K to constant weight. The main
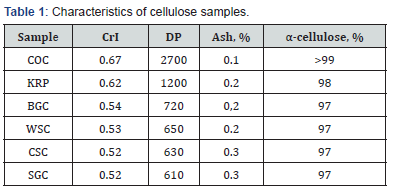
characteristics of the samples (crystallinity index CrI, degree of
polymerization DP, content of ash and α-cellulose) are shown in
the Table 1.
Samples of starch, pentosans, hexosans, Abietic Resin Acid
(ABA) and Triglycerides of Stearic (TGS), palmitic (TGP), were
supplied from Sigama-Aldrich. Klason lignin was isolated from
various biomasses using standard TAPPI method T222. Powdered
polyolefins were supplied from Ineos Olefins and Sigma-Aldrich.
Pelletization
The ground biomass was blended with powdered polyolefin,
and the mixture was compacted under pressure 50 MPa at
temperature of 445-450K for 1-2min. For comparison, the
pelletized biomass was also prepared under the same conditions.
Characterization of samples
Chemical composition of biomass samples was studied by
standard NREL methods [21]: LAP-001 (total solids), LAP-002
(cellulose and hemicelluloses), LAP-003 (lignin), LAP-005 (ash)
and LAP-010 (extractives). Bulk density was tested by standard
method ASTM E-873. Average degree of polymerization (DP) of
cellulose was measured by the viscosity method using diluted
cellulose solutions in Cadoxen [22]. Index of cellulose crystallinity
(CrI) was estimated using Jayme and Knolle method [23].
Combustion calorimetry
Combustion of samples in pelletized form (cca. 1g) was
carried out in a bomb calorimeter Parr-1341 at oxygen pressure
of 3 MPa with 1ml of added deionized water. The temperature and
its rise (ΔT ) were measured with accuracy ±0.001 K. The value of
energy equivalent of the calorimetric system (C) was determined
by combustion of standard benzoic acid. The correction of internal
energy change (ΔcU) for ignition (e1), as well as for formation and
dissolution of nitric acid (e2) were taken into account:
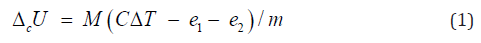
where m is true mass of sample, M is molecular mass of
substance or repeat unit of polymer.
To obtain the standard value of specific energy of combustion
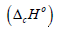 the Washburn’s correction was introduced. When the
standard enthalpy of combustion
the Washburn’s correction was introduced. When the
standard enthalpy of combustion 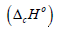 is calculated, the
correction was introduced for change in number of moles of gases
before and after combustion. The standard enthalpy of formation
is calculated, the
correction was introduced for change in number of moles of gases
before and after combustion. The standard enthalpy of formation
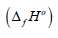 was calculated by the known equation:
was calculated by the known equation:
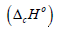 the Washburn’s correction was introduced. When the
standard enthalpy of combustion
the Washburn’s correction was introduced. When the
standard enthalpy of combustion 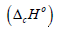 is calculated, the
correction was introduced for change in number of moles of gases
before and after combustion. The standard enthalpy of formation
is calculated, the
correction was introduced for change in number of moles of gases
before and after combustion. The standard enthalpy of formation
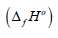 was calculated by the known equation:
was calculated by the known equation: 
where n(CO2) and n(H2O) are number of moles of CO2 and H2O
in combustion reaction; o
f Δ H (CO2, g) = -393.51 kJ/mol and o
f Δ H
(H2O, l) = -285.83 kJ/mol are standard enthalpies of formation of
carbon dioxide and liquid water.
For each sample three experiments were performed to obtain
the reliable TDC.
Results and Discussion
The results of calorimetric determination of standard
thermodynamic characteristics of individual components of plant
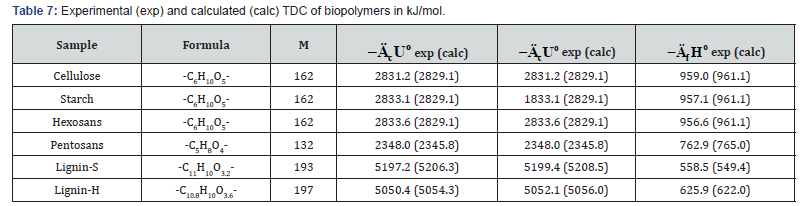
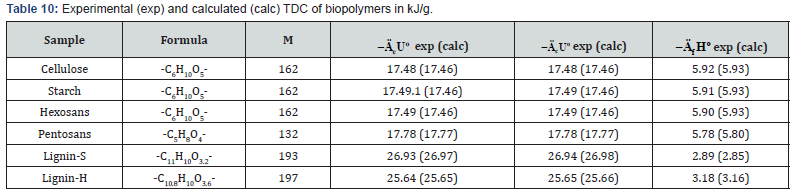
biomass are shown in Tables 2-5.
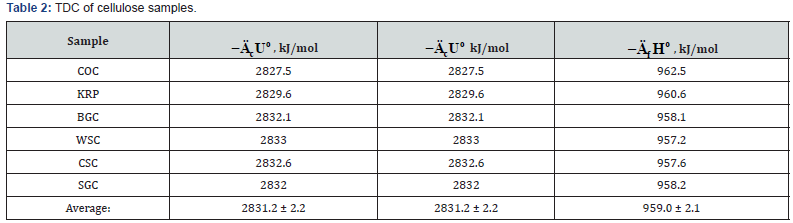


As can be seen from Tables 2 and Table 3, when determine
the TDC of carbohydrates the relative divergence between
combustion energies or enthalpies was small, about ±0.1%.
The obtained average values of TDC were in the range given in
literature [10,16,19,24].
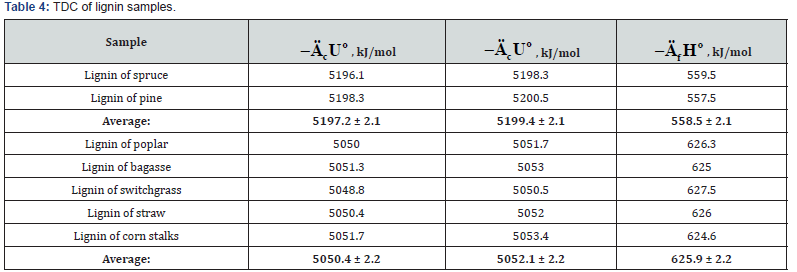
The study of lignin samples has shown (Table 4) that specific
energy of combustion for lignins of softwood were higher than
the corresponding values for lignins isolated from hardwood
(Lignin-S) and herbaceous plants (Lignin-H). The study of
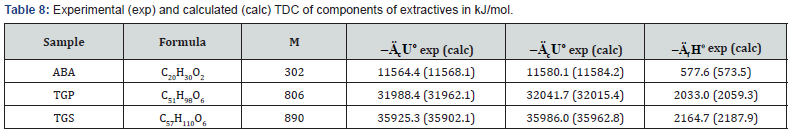
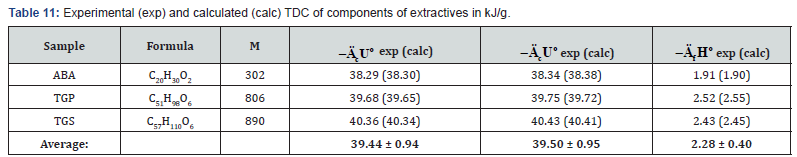
components of extractive substances (resin acids, e.g. ABA, and
various lipids) has shown that standard energy and enthalpy
of combustion was proportional to molecular mass of these
substances (Table 5).
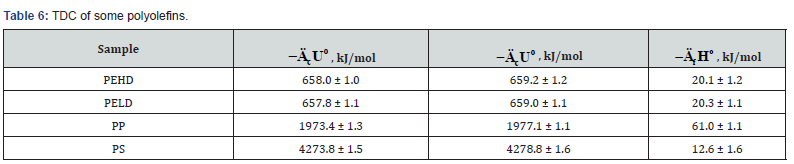
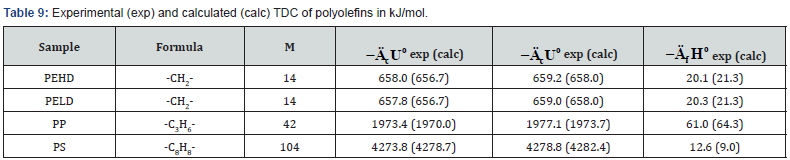
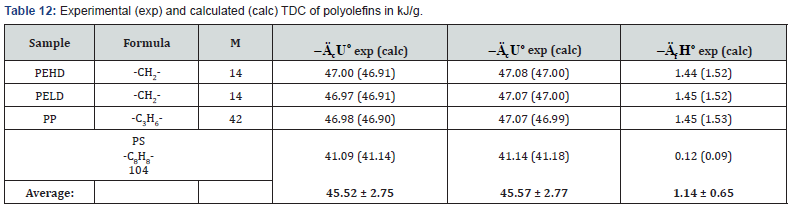
The determined TDC of some polyolefins (Table 6) are
confirmed by literature data [13,25].
In addition to experimental, there are also quite accurate calculating
methods to determine the TDC of individual biopolymers
of biomass, components of extractives, as well as some plastics
(Tables 7-9). For this purpose, the following equations can be
used [26]:
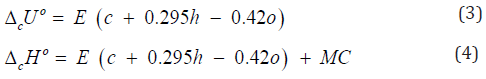
where E = -413 kJ/mol; c , h and o are number of atoms
C , H and O respectively in molecule of organic substance or in
repeat unit of polymer. ( ( ) 2 2 [ o MC n CO n O RT = −
is correction for
change of moles CO2 and O2 during combustion process, T o = 298
K is standard temperature.
The standard enthalpy of formation was calculated by
equation (2)
In many cases, the TDC are conveniently expressed in kJ/g
(Tables 10-12).
As can be seen from Tables 7-12, the calculated TDC are
quite close to experimentally characteristics determined using
a calorimetric bomb. Thus, the calculation method can be
successfully used for evaluation of TDC of organic components of
biomass, as well as of some plastics.
The results of compositional analysis of biomass samples by
standard NREL methods are shown in Table 13.

*Hemi denotes Hemicelluloses; Extr denotes Extractives.
The obtained TDC ( o )
i Y of individual components,
biopolymers and extractives (Tables 10 & 11), and their content
( ) i w in the biomass (Table 13) were used to evaluate the TDC (Y o )
of biomass sample according to additivity rule:

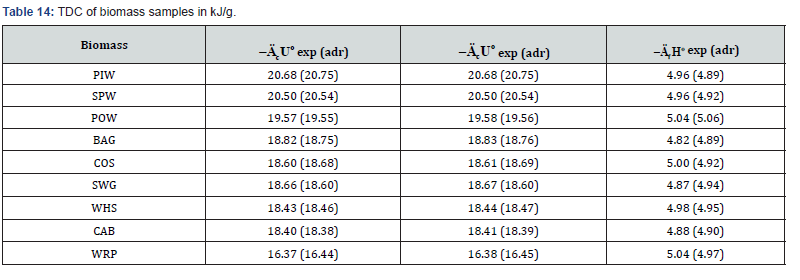
Besides, the pelletized fuels containing of biomass or
composite of biomass and mixed polyolefins (POL) were prepared.
The TDC of these fuels were determined experimentally (exp) or
calculated using the additivity rule (adr). The results revealed that
TDC of solid fuels calculated by equation (5) are confirmed by
experimentally obtained characteristics (Tables 14 & 15). These
data evidence on adequacy of the additivity rule to evaluate the
TDC of biomass-based solid fuels.

The study of pelletized fuels also showed that the densification
of initial biomass does not change the energy and enthalpy of
combustion, but increases the density of thermal energy (ED):

where d is density of solid fuel (kg/m3)
Additive of polyolefins into biomass samples increases the
energy and enthalpy of combustion, as well as the energy density
of the pelletized fuels (Table 15). As a result, energetic features
of biomass-based pellets become comparable with those of fossil
coals (Figure 1).
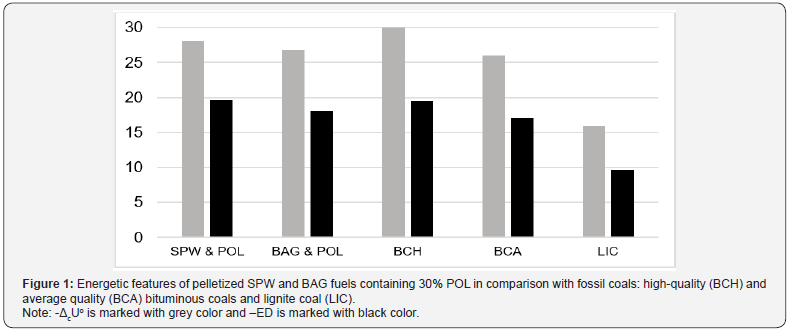
Figure 1 Energetic features of pelletized SPW and BAG fuels
containing 30% POL in comparison with fossil coals: high-quality
(BCH) and average quality (BCA) bituminous coals and lignite coal
(LIC)
Note: -ΔcUo is marked with grey color and –ED is marked with
black color
Conclusion
Solid fuels made of plant biomass and its compositions
with plastic additive were studied as an alternative to solid
fossil fuels such as coals. For this purpose, experimental and
calculation methods were used to determine the thermodynamic
characteristics (TDC) of individual components of plant biomass
and plastics. Using the results determined for individual
components, the TDC of various biomass samples and biomassplastic
blends were calculated by means of additivity rule. The
results revealed that calculated TDC of solid fuels were close to
experimentally obtained values. Thus, the obtained data evidence
the adequacy of the additivity rule to evaluate the TDC of solid
fuels based on biomass. It has been also found that fuel pellets
consisting of plant biomass and additive of plastics are the most
promising solid fuels, since they provide the higher energy and
enthalpy of combustion, as well as increased density of thermal energy than biomass only. Moreover, energetic features of
pelletized fuels become comparable with those of fossil coals.
For more articles in Academic Journal of Polymer
Science please click on:
https://juniperpublishers.com/ajop/index.php
https://juniperpublishers.com/ajop/index.php


Comments
Post a Comment