Recent Advances in Synthetic Applications of Polyvinylpyrrolidone Supported Reagents and Catalysts-Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER
SCIENCE









Abstract
This review summarized recent progresses in the
application of polyvinylpyrrolidone supported reagents and catalysts in
organic synthesis.
Keywords: Polyvinylpyrrolidone; Supported reagents; Supported catalysts; Multi-component reactions
Introduction
The use of solid-supported reagents and catalysts in
solution-phase chemistry has emerged as a leading strategy that exploits
the advantages of both solid- and solution-phase synthesis. The
approach essentially combines the benefits of product isolation and
purification in solid-phase synthesis with the high-speed development
and flexible choice of chemistry from the vast repertoire of solution
phase organic reactions. The organic molecules synthesis using
polymer-supported reagents and catalysts is highly attractive because
the work-up involves only simple filtration and evaporation of the
solvent [1]. Polyvinylpyrrolidone (PVP) is an amorphous polymer having
broad applications in biomedical field due to its special properties
such as low toxicity and good solubility in water and most organic
solvents, good adhesion characteristics, and great physiological
compatibility [2]. Also, PVP has good biocompatibility and has been
applied for many years as a biomaterial or additive to drug
compositions, e.g. as a blood plasma expander [3].
Polyvinylpolypyrrolidone (PVPP, crospovidone, or crospolividone) is a
highly cross-linked polyvinylpyrrolidone (PVP). The cross-linked form of
polyvinylpyrrolidone is insoluble in water, though it still absorbs
water and swells very rapidly generating a swelling
force. This property makes PVPP useful as a disintegrant in
pharmaceutical tablets [2]. Polyvinylpyrrolidone shows a strong binding
affinity to small molecules. Furthermore, its iodine complex,
povidon-iodine, is widely used as an anti-infective agent in clinical
treatments [4].
Polyvinylpyrrolidone Supported Reagents
A range of polyvinylpyrrolidone-supported reagents
has been developed for applications in organic synthesis. In general,
these reagents are employed in stoichiometric excess to drive the
reaction to completion. Simple filtration removes the spent resin from
the reaction solution and, thus, eliminates the need for any
time-consuming chromatographic work-up.

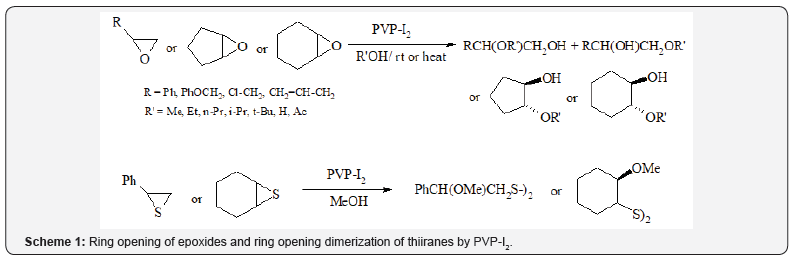
Iranpoor and coworkers [5] have prepared iodine supported
on polyvinylpyrrolidone (betadine) as catalysts for ring opening
of epoxides and as reagent for ring opening dimerization of
thiiranes in alcohols, water and acetic acid (Scheme 1). In this
report, the reaction of R-(+)-styrene oxide with I2 supported on
PVP in methanol was found to be very stereospecific and the
product isolated in 93% ee.

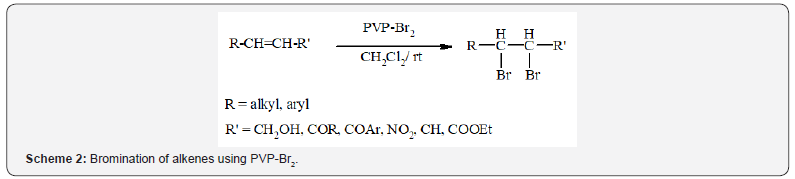
In another research, Lakouraj et al. [6] described the
preparation of polyvinylpyrrolidone-bromine complex (PVP-Br2)
as a mild and convenient reagent for selective bromination of
alkenes (Scheme 2) [6].
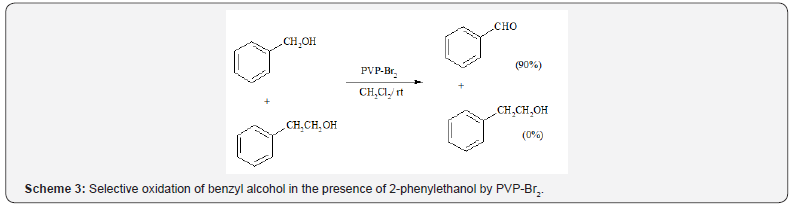
Selective oxidation of benzyl alcohol in the presence of
2-phenylethanol was also achieved at room temperature in the
presence of PVP-Br2 (Scheme 3).

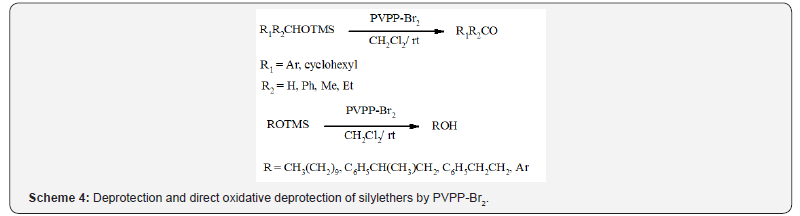
In the next research, Lakouraj and Mokhtary have developed
a convenient method for deprotection and direct oxidative
deprotection of silylethers to the corresponding hydroxy
and carbonyl compounds using polyvinylpolypyrrolidonebromine
complex (PVPP-Br2) (Scheme 4) [7]. Selective oxidative
deprotection of benzylic silyl ethers in the presence of primary
aliphatic alcohols was also achieved at room temperature.


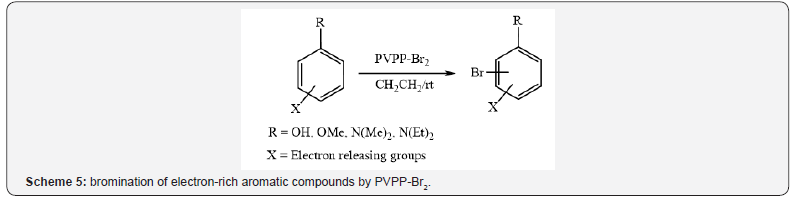
Also, PVPP-Br2 has been used for bromination of electron-rich
aromatic compounds [8]. The reaction proceeded smoothly with
phenols and N, N-alkylated amines to afford the corresponding
mono brominated product in good yields at ambient temperature
(Scheme 5).

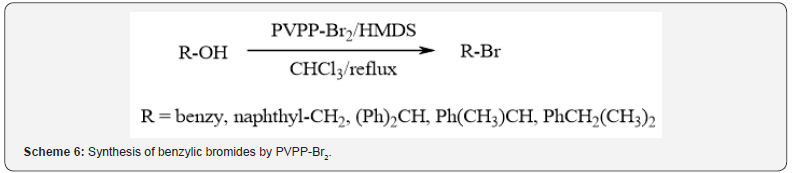
Furthermore, Mokhtary and Lakouraj synthesized benzylic
bromides in high yields by the reaction of the corresponding
alcohols with cross-linked polyvinylpyrrolidone-bromine complex
(PVPP-Br2) in the presence of hexamethyldisilane in chloroform
at reflux condition (Scheme 6) [9]. Selective conversion of benzyl
alcohol to benzyl bromide in the presence of 2-phenylethanol was
also achieved.
Surya Prakash et al. have prepared solid polyvinylpyrrolidonehydrogen
peroxide complex and used as solid hydroxylating
reagent [10]. This solid hydrogen peroxide is found to be much
safer, convenient and efficient reagent system for the ipsohydroxylation
of arylboronic acids to the corresponding phenols
in highyields at a faster rate (Scheme 7). The versatility of the
reagent has been further expanded for the one-pot synthesis of
halophenols.


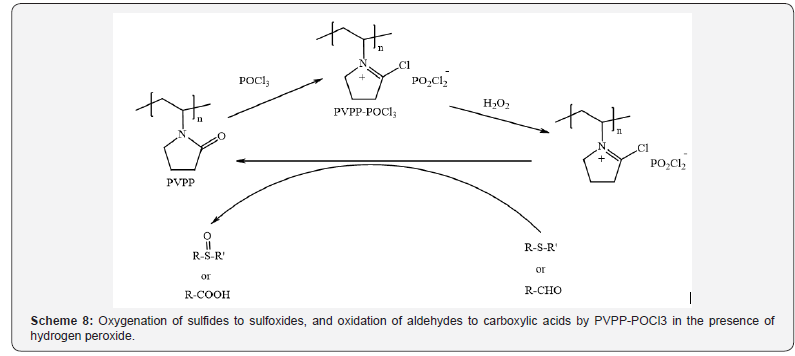
Lakouraj et al., [11] have demonstrated polyvinylpolypyrrolidone-
phosphorous oxychloride as a versatile polymeric Vilsmeier
reagent that exhibits excellent selectivity for oxygenation
of sulfides to sulfoxides, and oxidation of aldehydes to carboxylic
acids in the presence of hydrogen peroxide under mild reaction
conditions (Scheme 8) [11]. This polymeric Vilsmeier reagent was
found to retain its activity after months and is stable in a glass
bottle at room temperature.
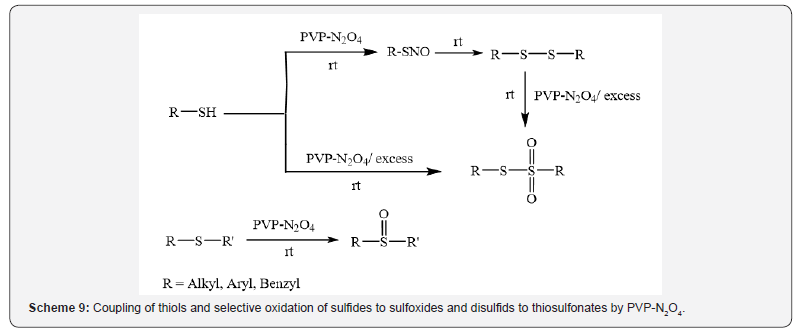
Iranpoor et al. [12] have reported dinitrogen tetraoxide
supported on polyvinylpyrrolidone (PVP-N2O4) as a nitrosating
and coupling agent for thiols and selective oxidation of sulfides to
sulfoxides and disulfids to thiosulfonates (Scheme 9) [12].


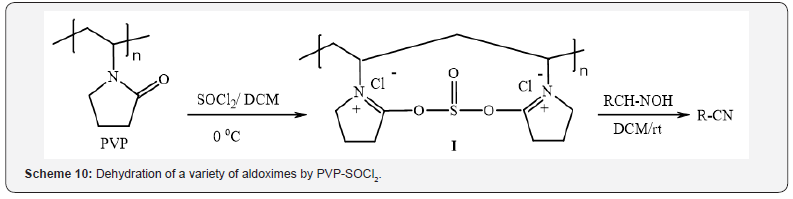
Tamami and Kiasat have reported [13] a polyvinylpyrrolidonethionyl
chloride complex by the reaction of thionyl chloride with
two equivalents of polyvinylpyrrolidone in dichloromethane at 0 ͦC.
The polymer-bound complex I was used for the rapid dehydration
of a variety of aldoximes to produce the corresponding nitriles in
high yields (Scheme 10).

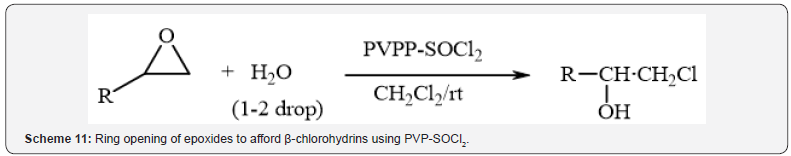
Also, Tamami et al. [14] have reported rapid ring opening
of epoxides to afford β-chlorohydrins with cross-linked
polyvinylpyrrolidone/thionyl chloride complex (PVP-SOCl2),
under mild reaction condition in high yields (Scheme 11).
Polyvinylpyrrolidone Supported Lewis Acidic Catalysts
Replacement of conventional, toxic and unstable Lewis acidic
catalysts with eco-friendly reusable solid acid catalysts is an
essential requirement in the development of green chemistry. For
example, boron trifluoride is widely used in organic syntheses as
a Lewis acid. However, boron trifluoride is highly water sensitive,
irritant, and has to be used in a carefully dried apparatus.
Moreover, all work must be carried out in an efficient fume hood,
and its recovery from the reaction mixture results in a main
source of waste, which on an industrial scale is environmentally
unacceptable.
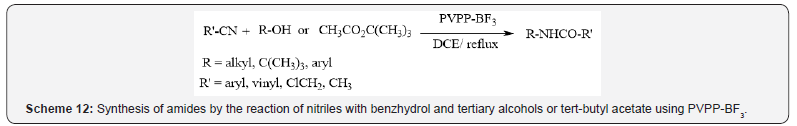
Mokhtary et al. have reported several papers on the application
of the polyvinylpolypyrrolidone-bound boron trifluoride (PVPPBF
3) as a stable polymeric Lewis acid catalyst in some organic
reactions such as synthesis of amides by the reaction of nitriles
with benzhydrol and tertiary alcohols or tert-butyl acetate via
Ritter reaction (Scheme 12) [15, 16], the acylation of alcohols,
phenols and trimethylsilyl ethers with acetic anhydride (Scheme
13) [17], the synthesis of coumarins via Pechmann condensations
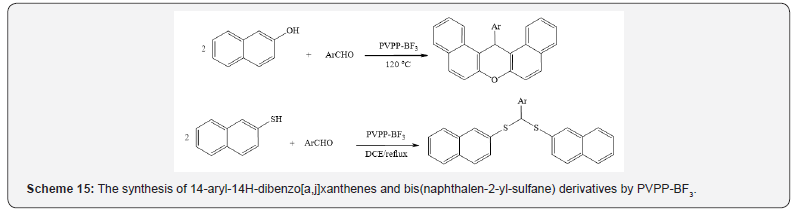
of phenols with ethyl acetoacetate (Scheme 14) [18], the synthesis
of 14-aryl-14H-dibenzo [a,j] xanthenes and bis(naphthalen-
2-yl-sulfane) derivatives (Scheme 15) [19], the synthesis of
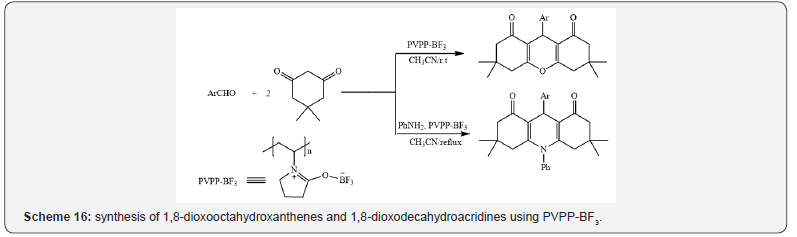
1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines
via condensation of aromatic aldehydes and dimedone in acetonitrile at room temperature, and aromatic aldehydes,
dimedone, and aromatic amines in acetonitrile at 80 °C
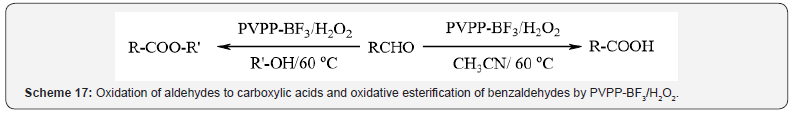
respectively (Scheme 16) [20], the oxidation of aldehydes to
carboxylic acids and oxidative esterification of benzaldehydes in
the presence of 35% hydrogen peroxide (Scheme 17) [21] and the
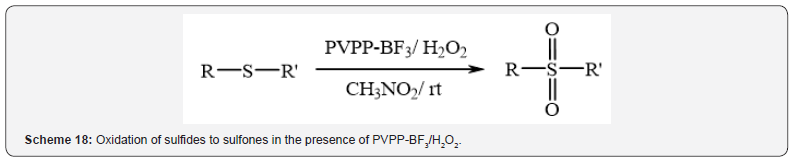
oxidation of sulfides to sulfones in the presence of 35% hydrogen
peroxide at room temperature (Scheme 18) [22]. Excellent yields,
easy work-up and reusability and stability of the catalyst are some
advantages of these methods.








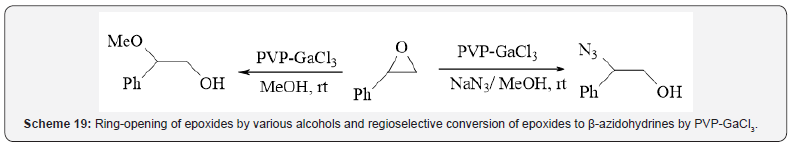
Pourali and et al. prepared cross-linked polyvinylpyrrolidone
supported GaCl3 for efficient and regioselective ring-opening
reaction of epoxides by various alcohols under solvent-free
conditions at room temperature (Scheme 19) [23]. Furthermore,
regioselective conversion of epoxides to β-azidohydrines was
accomplished by sodium azide in MeOH in the presence of GaCl3/
PVP at room temperature. GaCl3/PVPP is a non-hygroscopic and
recoverable catalyst and is easily separated from reaction mixture
by a simple filtration and reused repeatedly

In the next research, an efficient synthesis of chromenylphenylpropanone
derivatives as warfarine-like analogues was
developed by the Michael addition of 4-hydroxycoumarin to α,
β-unsaturated compounds in the presence of polyvinylpolypyrrolidone
supported antimony (III) chloride (PVPP-SbCl3) as a new
polymeric Lewis acid catalyst in chloroform at reflux conditions
(Scheme 20) [24].
Polyvinylpyrrolidone Supported Brönsted Acidic Catalysts
Polymer-supported Brönsted acidic catalysts have gained
considerable importance due to their low cost, high efficiency, easy
work-up, and reusability. Ghorbani-Choghamarani et al. reported
trimethylsilylation and formylation of alcohols in the presence ofpolyvinylpolypyrrolidoniume tribromide in acetonitrile at room
temperature (Scheme 21) [25].
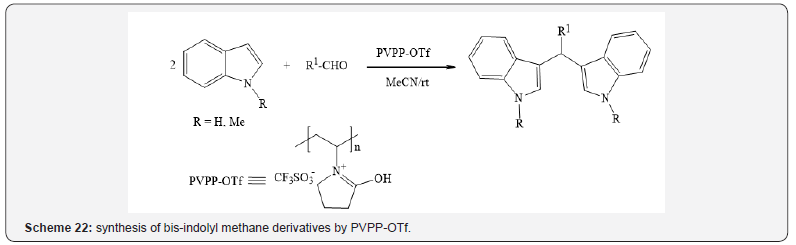
Khaksar et al. have prepared polyvinylpolypyrrolidonesupported
triflic acid (PVPP.OTf) as an environmentally friendly
and efficient catalyst for the synthesis of bis-indolyl methane
derivatives by the reaction of indole or N-methyl indole with
aldehydes (Scheme 22) [26].


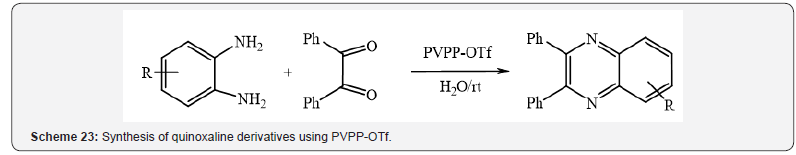
Furthermore, PVPP.OTf was found to be useful as a recyclable
heterogeneous catalyst for the rapid synthesis of quinoxaline
derivatives (Scheme 23) [27].
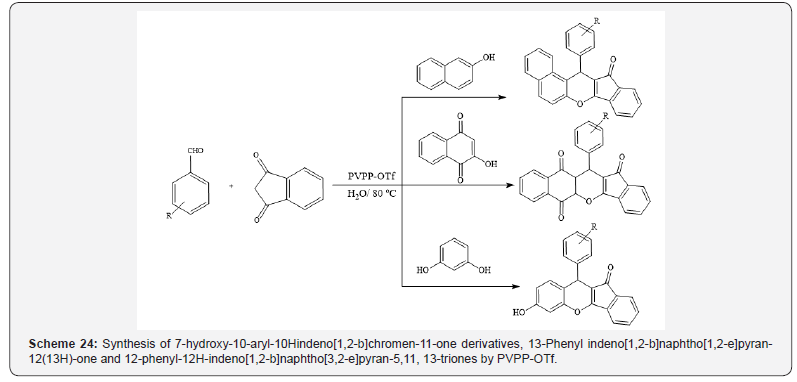
Also, polyvinylpolypyrrolidone-supported triflic acid has been
used as a recyclable catalyst for synthesis of a series of 7-hydroxy-
10-aryl-10Hindeno[1,2-b]chromen-11-one derivatives, 13-phenyl
indeno[1,2-b]naphtho[1,2-e]pyran-12(13H)-one and 12-phenyl-
12H-indeno[1,2-b]naphtho[3,2-e]pyran-5,11, 13-triones (Scheme
24) [28].

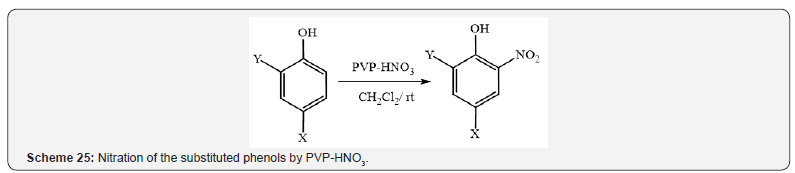
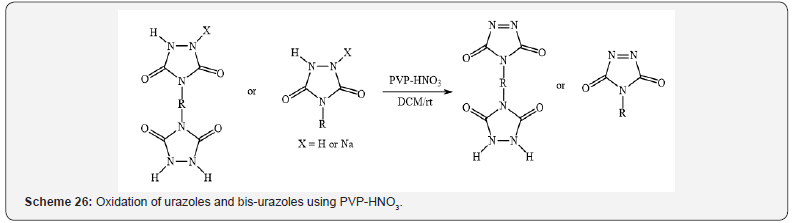
Nitration of the substituted phenols (Scheme 25) [29] and oxidation
of urazoles and bis-urazoles to the corresponding triazolinediones
(Scheme 26) [30] were reported by Nikoorazm et al.
and Ghorbani-Choghamarani et al. respectively in dichloromethane
at room temperature using supported nitric acid on polyvinylpyrrolidone
as an efficient, environmentally friendly, mild catalyst.



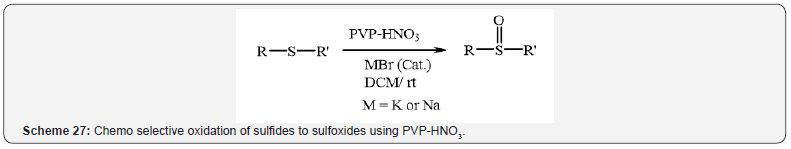
Furthermore, Zolfigol et al. was described chemo selective
oxidation of sulfides to sulfoxides using PVP-HNO3 in the presence
of a catalytic amount of KBr or NaBr (Scheme 27) [31].
The synthesis of xanthenes derivatives including 1,8-dioxooctahydroxanthenes,
14-aryl-14H-dibenzo[a,j] xanthenes, and
12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones reported
by Shirini et al, using O-sulfonated poly(4-vinylpyrrolidonium)
chloride [PVP-SO3H]Cl as a polymeric solid acid catalyst (Scheme
28) [32].



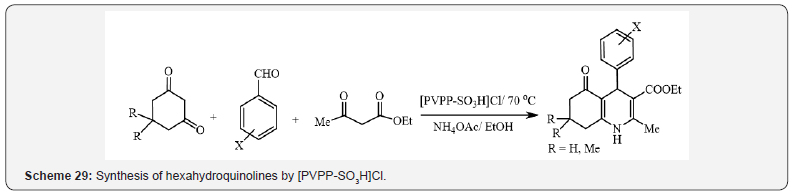
Also, polyvinylpolypyrrolidone supported chlorosulfonic acid
([PVPP-SO3H]Cl) was evaluated by Mokhtary et al, as a recoverable
catalyst for the one-pot synthesis of hexahydroquinolines (Scheme
29) [33], dihydropyrimidinones and octahydroquinazolin-2,5-
diones (Scheme 30) [34].
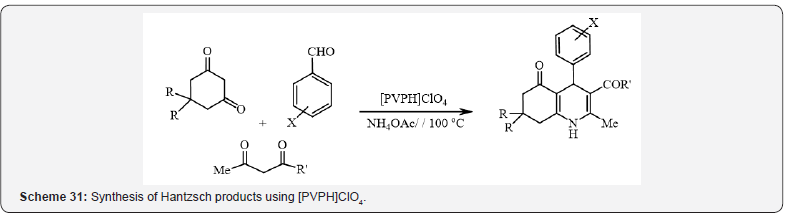
In another research, Abedini et al. have described a green
approach for the promotion of the synthesis of Hantzsch products
using polyvinylpyrrolidinium perchlorate ([PVPH]ClO4) as a new
modified polymeric catalyst (Scheme 31) [35].


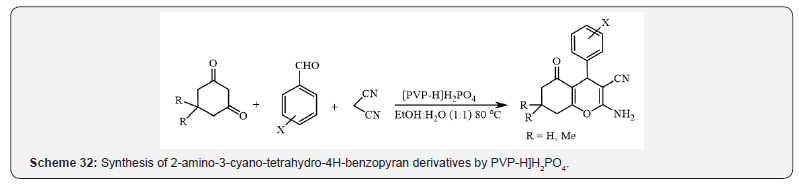
In the next research, Shirini et al., have introduced
polyvinylpyrrolidonium hydrogen phosphate ([PVP-H]H2PO4) as a
heterogeneous, and reusable catalyst for the synthesis of 2-amino-
3-cyano-5-oxo-5,6,7,8-tetrahydro-4H-benzopyrans (Scheme 32)
[36].
In another study, sulfuric acid-modified polyvinylpyrrolidone
([PVP-SO3H]HSO4) was prepared by Safaei et al. as an efficient
reusable polymeric catalyst for the one-pot multi component
synthesis of acridinedione derivatives as an important class of
heterocyclic compounds (Scheme 33) [37].

Polyvinylpyrrolidone Supported Pd Catalyst
A series of poly(N-vinyl-2-pyrrolidone) immobilized Pd
nanoparticles (PVP-Pd) with varying particle size have prepared
by Li et al., [38] using the stepwise growth reaction. The effect of
Pd particle size on the Suzuki reaction between phenylboronic acid
and iodobenzene was investigated by the use of four Pd catalysts
(Scheme 34). The catalytic activity of the Pd nanoparticles
expressed in terms of the initial turnover frequency (moles of the
biphenyl product per mole of total surface Pd atoms per min) was
found to be in the order of Pd (3.9nm) > Pd (3.0nm) ~ Pd (5.2nm)
> Pd (6.6nm), indicating that surface Pd atoms do not all have the
same reactivity in this reaction. The general trend of increased
catalytic activity with the decrease in the particle size suggests
that the low-coordination number vertex and edge atoms on the
particle surface are active sites for the Suzuki reaction. The lower
catalytic activity for the smallest Pd nanoparticles may be due to
stronger adsorption of the reaction intermediates on the particle
surface, in which the strongly adsorbed species act as a poison to
the reaction thereby decreasing the rate of the reaction.


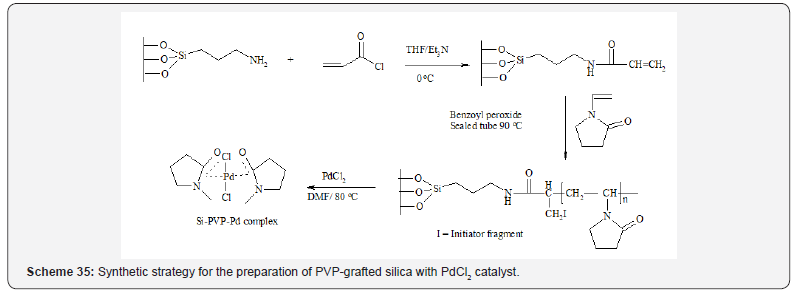
In another work, Tamami et al., [39] was prepared a catalytic
system based on palladium nanoparticles supported on poly(Nvinylpyrrolidone)
grafted silica (Scheme 35).
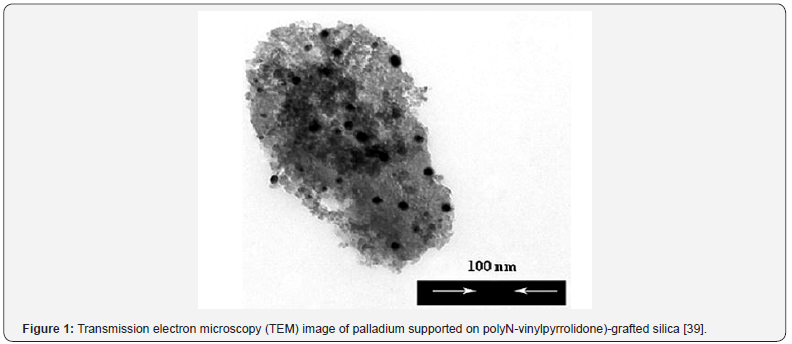
The complexation of PVP-grafted silica with PdCl2 was carried
out to obtain the heterogeneous catalytic system. Transmission
electron microscopy (TEM) image showed that palladium
dispersed through the support in nanometer size (Figure 1).


This catalytic system exhibited excellent activity in crosscoupling
reactions of aryl iodides, bromides and also chlorides
with olefinic compounds in Heck-Mizoraki reactions in short
reaction time and high yields (Scheme 36).
Conclusion
Polymer-supported reagents and catalysts have emerged as
important tools for the rapid preparation of chemical compounds
in solution-phase. Clean methodologies, easy preparation of
the catalysts, simple work-up procedures, good to high yields,
environmentally friendly and reusable catalysts are some
advantages of polyvinylpyrrolidone supported catalysts and
reagents in organic multi-step synthesis. Further progresses in
the development of new PVP-bound reagents, and catalysts will
continue to attract innovative application of this strategy in multicomponent
synthesis.
Acknowledgements
Financial support by Rasht Branch, Islamic Azad University is
gratefully acknowledged.
For more articles in Academic Journal of Polymer
Science please click on:
https://juniperpublishers.com/ajop/index.php
https://juniperpublishers.com/ajop/index.php


Comments
Post a Comment