Polyurethane Acrylate/Montmorillonite Nanocomposites-Juniper Publishers
JUNIPER PUBLISHERS- ACADEMIC JOURNAL OF POLYMER
SCIENCE
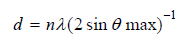

Abstract
In order to create polymer nanocomposites with high
performance on the basis of polyurethane acrylates (PUA) with
montmorillonite (MMT), three methods of chemical modification of the
layered silicate surface have been developed. The first modification
method is based on using of two different functional modifiers
(organophilic and reactive), the second method is based on modification
with synthesized by us compound which contains urethane groups, and the
third one in based on using synthesized by us modifier containing
urethane and reactive groups. Exchange capacity of the MMT surface was
determined by adsorption of indicator “methylene blue”. Intercalation of
modifier into the interlayer space of MMT was confirmed by X-ray
analysis; the content of organic component in the modified MMT (MMT/M)
was determined by thermogravimetric analysis. The resulting organoclay
is purposed for the formation of nanostructured composites based on
cross-linked polyurethane acrylates with improved physical and
mechanical properties. The obtained polyurethane acrylate nanocomposites
with different type MMT/M exhibit the increased in 1.6 - 2.6 times
tensile strength as compared to original polymer matrix. WAXS method has
proved an intercalation of modifier into MMT interlayer space
(increased distance between layers after modification), as well as the
total exfoliation of MMT in PUA matrix, characterized by the
disappearance of the absorption peak which is responsible for layered
structure.
Keywords: Montmorillonite; Modification; Polyurethane acrylate; Nanocomposites
Abbrevations:
CTAB: Cetylammonium Bromide; DMF: Dimethyl Formamide; DMAEMAC:
Dimethyl Ammonium Ethyl Methacrylate Chloride; DTA: Differential
Thermogravimetric Analysis; HMDI: 1,6-Hexamethylene Diisocyanate; OUA:
Oligourethane Acrylate; OUAC: Oligouretane Amonium Chloride; OUMAAC:
Oligourethane Methacrylate Ammonium Chloride; POTMG-1000: Poly Oxytetra
Methylene Glycol with Molecular Weight of 1000; NMDEA: N-Methyldiethanol
Amine, Na-MMT; Na-form of Montmorillonite; MB: Methylene Blue; MDI - 4:
4´- Diphenylmethane Diisocyanate; MW: Molecular Weight; MWD: Molecular
Weight Distribution; MMT: Montmorillonite; MMT/M: Modified
Montmorillonite; PP: Prepolymer; PUA: Polyurethane Acrylates; WAXS:
Wide-Angle X-ray Scattering
Introduction
Lately polymer-based nanocomposites are of great
interest both in science and industry due significant improvement of
performance of their properties compared to initial polymers and
conventional macrocomposites. Polymer based nanocomposites are
characterized by increased strength and heat resistance as well as
reduction of gas permeability and flammability [1]. Creation of
polyurethane acrylate (PUA) - organoclay based nanocomposites with high
performance properties is a real chance to improve the basic properties
of initial PUA polymer matrix. The increase of the strength and
durability of PUA based materials may be achieved by incorporation of
organoclay nanoparticles into the polymer matrix [2]. In order to modify
the natural MMT by ion exchange method, it should be converted to the
sodium (rarely to potassium) form by treatment with alkali metals
carbonates [3], since the alkali metal cations are easier replaced on
the organic cations compared to alkaline-earth cations of natural MMT.
In order to create polymer based on There are the following ways of MMT
modification used in
the field of polymer nanocomposites based on polar heterochain polymers:
a) modification with cationic surfactants (ammonium and phosphonium cations) [4].
b) modification with reactive compounds [2,5].
c) modification with monomers during the reaction of
polycondensation followed by the chain growth in the interlayer space
[1,6].
A disadvantage of the first method is nonpolar
nature of the used surfactants. As a result of different nature of the
organic modifier and polymer matrix the particles of modified MMT form
an agglomerate that do not contribute to the intercalation and
exfoliation of polyurethane into the interplanar space of MMT.
Disadvantages of the second method are a small molecular size of
reactive modifiers’ molecules which does not provide a sufficient
organophilicity of the mineral surface that complicates the penetration
of the monomer molecules into the interplanar space. The same drawbacks
are observed in case of the third
modification method, namely modification with diamines, one
of the amino groups of which is in a salt form and it is capable
to ion exchange reactions in interplanar space of MMT. The sizes
of the molecules of these monomers are not large enough to
provide the sufficient organophilicity of MMT to overcome the
agglomeration of the particles.
In order to create polymer nanocomposites with high
performance on the basis of polyurethane acrylates (PUA) with
montmorillonite (MMT), three methods of chemical modification
of the layered silicate surface have been developed. The first
modification method is based on combined modifying of MMT
with two types of modifiers: cationic surfactant (cetylammonium
bromide), which imparts organophilicity to MMT surface and
facilitates dispersion of the mineral in organic medium; and a
bifunctional amine - dimethylaminoethyl methacrylate. The
developed method of MMT functionalization alows to obtain
an organophilisized minerals containing reactive groups of
different nature (acrylate, hydroxy, amine) on its surface, capable
to participate in reactions of photoinitiated polymerization and
polycondensation [7], which should increase the physical and
mechanical properties of polymers. The second method of MMT
modification lies in use of ammonium ions as surfactants, which
include the urethane groups - oligouretane ammonium chloride
(OUAC). Its molecule is sufficiently sterically large for provide
the intercalation and exfoliation of MMT particles in polar
organic media [8,9]. The new approach of MMT modification
in contrast to the classical use of surfactants composed of ionic
group and aliphatic large fragment (C12 - C20), consists in the
use of synthesized by us surfactants with urethane groups in
their structure. MMT modified with such cationic surfactants can
form stable hydrogen bonds with polymer matrix: polyurethane,
polyamide, polyamide, etc. Hydrogen bonding between the
modified surface of MMT and polyurethane macromolecules
provides a complete exfoliation of the modified MMT particles
and strong physical interaction between inorganic and organic
components. The high affinity of the modified nanofillers with
polymer matrix provides the increased strength of polyurethane
and other polar polymers-based materials. The third method.
In order to create the nanofiller which form both physical
and chemical bonds with polymer matrix, the new modifier
oligouretane methacrylate ammonium chloride (OUMAAC)
having both urethane and reactive methacrylate groups was
synthesized [10,11]. The new modifier was synthesized by
analogy with above mentioned OUAC.
Reactive methacrylate groups form chemical bonds with
oligourethane acrylate matrix during the in situ polymerization.
Modified surface of MMT nanoparticles, exfoliated due to the
presence of urethane groups, provides not only physical but also
chemical affinity with polymer matrix. Physical and chemical
bonds of nanofiller with polyurethane acrylate matrix provides
a significant increase of polymer nanocomposite service
properties as compared to the initial polymer matrix.
Materials and Methods
We used a natural montmorillonite from clay deposit
“Askaniya” (Georgia). MMT modifiers: cetylammonium bromide
(CTAB) [Aldrich] and dimethylammoniumethylmethacrylate
chloride (DMAEMAC) [Aldrich]. N-methyldiethanol amine
(NMDEA) [Aldrich]; 1,6-hexamethylene diisocyanate (HMDI)
[Aldrich] were used for synthesis of oligouretanamonium
chloride (OUAC) nanomodifier. Isopropyl alcohol was distilled
in the presence of anhydrous calcium chloride and fraction
with bp = 82oC was isolated. For the synthesis of OUMAAC
nanomodifier, the N-methyldiethanol amine (N-MDEA)
[Aldrich], 1,6-hexamethylene diisocyanate (HMDI) [Aldrich],
hydroxyethyl methacrylate (HEМА) [Aldrich] were used.
Polyoxytetramethylene glycol with molecular weight of
1000 (POTMG-1000) [Aldrich], 4, 4´- diphenylmethane
diisocyanate (MDI) and HEМА [Aldrich] were used for synthesis
of polyurethane acrylate (PUA). 2- propylolphenyl keton
(Darokur-1173) was used as a photoinitiator of polymerization.
To determine the exchange capacity of MMT the indicator of
methylene blue (MB) (ZAT “Khimservice”, Russia) was used.
Preparation of the sodium form of MMT
The suspension of original MMT (5%) in distilled water
boiled for 1 hour with sodium carbonate in a weight ratio of
MMT: sodium carbonate = 100:1 was used for obtaining of Naform
of MMT (Na-MMT). The fourfold centrifugation, followed by
washing with distilled water, was used to separate the resulting
Na-MMT from the solution of sodium carbonate. The content of
dry matter in Na-MMT suspension was defined gravimetrically.
Modified MMT/M was then obtained using the resulting Na-
MMT suspension.
Exchange capacity of Na-MMT
Exchange capacity of the MMT surface was determined
by adsorption of indicator “methylene blue” onto Na-MMT
surface using photocalorimetry analysis [12] for calculation
the modifier: MMT ratio. To determine the exchange capacity of
MMT the estimation of adsorption of MB on the MMT surface
was carried out, i.e. the dependence of the adsorption value on
equilibrium MB concentration (the concentration of the solution
on reaching the adsorption equilibrium). For that the eight
samples of MMT with the same mass were filled up with the
solutions of the same volume but different concentrations of MB.
The equilibrium concentration was determined in three days.
The adsorption (α, mmol/g) was calculated as the difference
between the initial amount of MB added to MMT suspension in
solution with concentration C0 and the amount of MB remaining
after adsorption onto 1g of montmorillonite according to:
where: C0 - initial concentration of MB;
Cp - equilibrium concentration of MB (per 1 g of MMT) after
adsorption;
V - volume of an aqueous sample solution, l;
g - mass of the MMT sample.
The equilibrium concentration increases sharply after
adsorption raises more than 1.5mmol/g. That mean that exchange
capacity of MMT used amounts to 1.5 [13]. The concentration of
MB in solution was measured by photocolorimeter CK-2PM (PO
“ZOMZ”, Russia) after the separation of aqueous solution from
the MMT.
X-ray diffraction
The polymer structure in the molecular level was studied
by the wide-angle X-ray scattering technique (WAXS) using a
DRON-4-07 diffractometer; X-ray optic scheme was arranged
to operate in the transmission mode of the Debye-Scherrer
method. CuK α-radiation used as produced by an anode X-ray
generator was monochromatized with a Ni-filter. The details of
WAXS are presented in [14]. Scattered X-rays were detected with
a scintillation counter in an automated stepwise scanning mode.
The measured values of scattering intensity were corrected
for attenuation of the incident X-ray beam by tested samples
and subsequent deviation of the intensity of background X-ray
scattering by the collimator system. The values of scattered
intensity were normalized to the scattering volume. Finedispersed
powders of ММT and MMT/M were placed into the
cells. Registration of scattered intensity was carried out under
condition of step-type scanning of scintillation detector in the
scattering angles range from 2 to 400. The distance (d) between
layers of particles in the MMT was determined from Bragg’s
equation [15]:
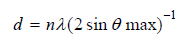
where n - the serial number of the diffraction peak in the
diffraction patterns (n = 1), λ - wavelength of the characteristic
X-rays (for CuKα λ = 0,154nm), θ - angle of X-ray scattering.
IR-spectroscopy
Samples of modifier were obtained on irtran substrate by
pouring from an aqueous solution. FTIR spectra were measured
using infrared Fourier transform spectrometer «Tensor-37»
Bruker / FT-IR-Spectrometer (Germany) in the range of wave
numbers of 4500-500cm-1. The assignment of the bands was
performed according to [16].

Mechanical testing
The tensile device FU-1000 (VEB MWK “Fritz Heckert”,
Germany) was used. The tensile speed was 100mm min-1
and temperatue 25 0C. For each measurement we used three
samples. Samples were prepared in a form of strips (width - 4
mm, operating length - 2mm). Measurements were carried out in
accordance with Standard 14236-81; allowed error - 3%.

Differential thermogravimetric analysis
Differential thermogravimetric analysis (DTA) was carried
out using a Q-1000 derivatograph, (MOM, Budapest) in the air
under the following conditions: average heating rate -10 deg
min-1; temperature range: 20-10000C; the weight of samples:
100mg; inert substance: Al2O3; sample holder: a ceramic conelike
crucible.
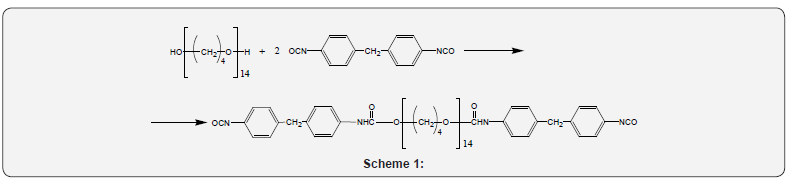
Prepolymer synthesis
In order to obtain the prepolymer (PP) the initial components
were placed into a three-necked reactor, equipped with inputoutput
device of inert gas (argon) in amount corresponding to
the ratio of their functional groups - NCO:OH = 2:1. The resulting
mixture was heated under the continuous stirring at 80 - 850C
(30min), followed by cooling the reacting mixture to 150C. The
estimated content of isocyanate groups in the PP amounted
to 6.3 ± 0.1 %wt. Scheme of PP synthesis is presented below:
(Scheme 1)
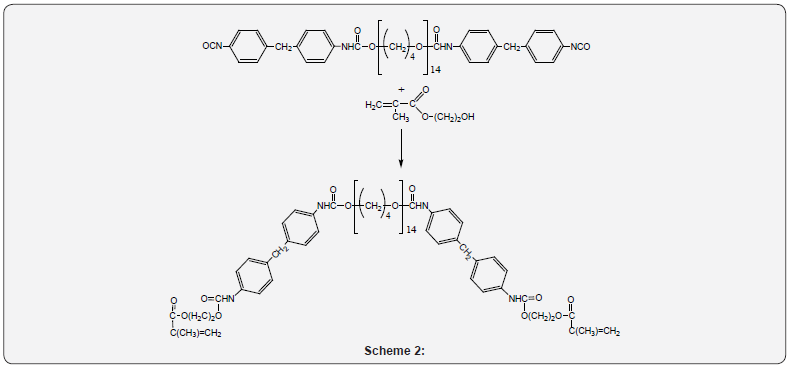
Synthesis of oligourethane acrylate (OUA)
OUA was synthesized using MDI based PP. The calculated
amount of HEMA in dimethyl formamide (DMF) was added to
PP at the temperature of 55 0C under continuous stirring; the
NCO:OH ratio = 1:1. The process of OUA formation took no more
than 1h under the continuous stirring at 55 0С.
Scheme of OUA preparation: (Scheme 2)
Results and Discussion
Modification of Na-MMT with different types of modifiers
The joint adsorption of components was studied using the
method of two-phase titration with sodium lauryl sulfate in
the presence of indicator MB, which was used for the selective
determination of the concentration of the surfactant CTAB [12],
which allows to determine the content of CTAB - the only one
cationic component, which is a classical surfactant.

In order to study the joint adsorption, the weighted amount
of Na-MMT suspension was mixed with solutions of CTAB and
DMAEMAC (with different ratio of the latter). The amount of
CTAB and DMAEMAC was equivalent to exchange capacity of
MMT (1.5mmol/g). To determine the equilibrium concentration
of CTAB, the titration of the solution was carried out after
attaining of adsorption equilibrium (in three days).
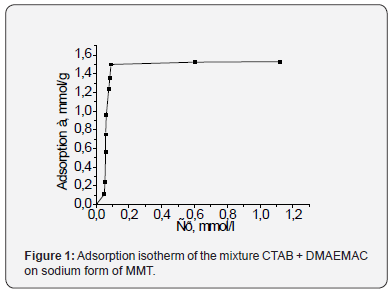
Two-phase titration of solutions after attaining of adsorption
equilibrium showed that CTAB adsorbed on the Na-MMT
regardless of DMAEMAC content in solution in an amount which
corresponds to the exchange capacity of MMT (1.5mmol/g).
Adsorption isotherms of CTAB mixed with DMAEMAC have the
same character as in the absence of the latter (Figure 1).

Thus, it has been found experimentally that CTAB as strong
cationic surfactant capable to displace the DMAEMA ions from
the montmorillonite surface. Therefore, modification with CTAB
was carried out in an amount that corresponds to half of the
ion exchange capacity of the mineral, and DMAEMAC addend
was carried out with 30% excess per the residual ion exchange
capacity, which was 50% of the initial ion exchange capacity of
the mineral. To prove the simultaneous co-adsorption of both
CTAB and DMAEMAC on the Na-MMT it was necessary to find
a way of detection of DMAEMAC ions in aqueous solution. For
this purpose, we used FTIR spectroscopy. Based on the fact that
determination of the low concentrations of DMAEMAC using
FTIR spectroscopy is not possible, we decided to analyze the dry
residues of salts’ aqueous solutions on irtran. FTIR data have
shown, that dry residues of DMAEMAC solutions have a visible
absorption band with maximum at 2497cm-1 and 2532cm-1 [16],
corresponding to NH+ of alkylammonium. Therefore, to prove
the joint adsorption the weight amount of Na-MMT suspension
was poured with a solution of CTAB and CTAB DMAEMAC in the
ratio: CTAB - 0.75mmol/g MMT and DMAEMAC - 0.75mmol/g
MMT, respectively. Preliminary we obtained the FTIR spectrum
of dry residue of DMAEMAC solution which was added to MMT.
It can be clearly seen the absorption bands with maxima at
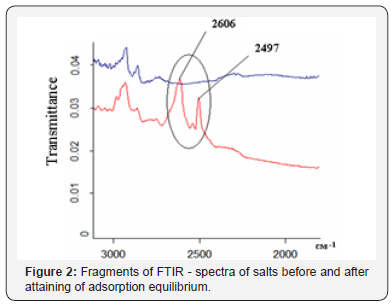
2497cm-1 and 2532cm-1 (Figure 2). After attaining of adsorption
equilibrium (in three days) the FTIR spectrum of the dry residue
of the solution displayed a complete absence of absorption bands that corresponded DMAEMAC. Thus, the adsorption
of DMAEMAC was confirmed by comparative analysis of the
FTIR spectra of salt solutions “before” and “after” attaining of
adsorption equilibrium (Figure 2).
Absorption band with the maximum at 2606cm-1 in the FTIR
spectra (Figure 2) of dry residues of initial solution of amine salts
indicates the presence of NH+ of alkyl ammonium cation (bands
at 2497cm-1 and 2532cm-1) whereas in sample after installing
the adsorption equilibrium these bands are not found. MMT
modification was carried out by adding the modifier solutions
(CTAB and DMAEMAC) of preset concentration to aqueous Na-
MMT dispersion. After adding CTAB and DMAEMAC solutions the
resulting mixture was diluted with distilled water to a ratio of 1g
MMT per 500ml of water. Modification was carried out for 48
hours, whereupon the precipitate of modified MMT was filtered,
dried in an oven at 60 °C till constant weight and crushed in an
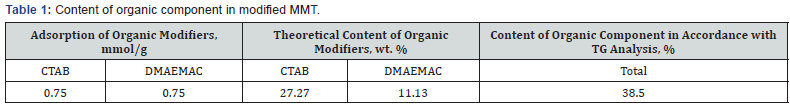
agate mortar and in a ball mill. The content of organic component
in modified montmorillonite was evaluated using TG data, (Table
1) as the difference between the mass of the sample “before”
and “after” thermo destruction with regard to moisture content
(0.5%wt.). The content of organic component in the modified
montmorillonite - amounts to 0.75mmol/g for each of the two
components and corresponds to the value of exchange capacity
of montmorillonite surface.

Modification of Na-MMT with oligouretane ammonium chloride (OUAC)
Based on the fact that chemical modification of MMT is
carried out predominantly with aqueous solutions of cationic
surfactants with a concentration below the critical micelle
concentration, we have proposed a scheme for the obtaining
of cationic surfactant (OUAC) solution, with a concentration
of 4x10-3 mol/l. OUAC synthesis was described in previous
publication [13]. The structural formula of OUAC: (Scheme 3)

n=1-3
Modification of MMT was carried out by addition to Na-MMT
suspension of the solution of OUAC with 50% excess relative to
calculated exchange capacity of Na-MMT. After adding an OUAC
solution the resulting mixture was diluted with distilled water to
a ratio of 1g MMT per 500ml of water. Addition of OUAC solution
to the MMT suspension resulted in instantaneous coagulation
of MMT particles followed by formation of a white precipitate.
Filtration, drying and grinding of the modified MMT was carried
out by analogy with the previous method. To assess the content
of the organic component in the modified MMT, the TGA study
was conducted. TGA data showed that the organic part content
was about 40% that substantially corresponds to its theoretical
content.
Swelling of modified MMT in organic solvents
Swelling study of modified MMT in organic solutions showed
that the modified MMT formed a stable gel in aprotic organic
solvents such as dimethyl formamide and dimethyl sulfoxide. Gel
formation indicates a high degree of solvent intercalation into
the interlayer space of MMT and physical network formation.
Modification of Na-MMT with oligouretane methacrylate amonium chloride (OUMAAC)
Preparation of cationic surfactant OUMAAC solution with a
concentration of 5x10-3mol/l was carried out similarly to OUAC.
OUMAAC Synthesis was described in previous publication [17].
The MMT OUMAAC modification was carried out similarly to the
OUAC modification. The structural formula of OUAC: (Scheme 4)
n=1-3

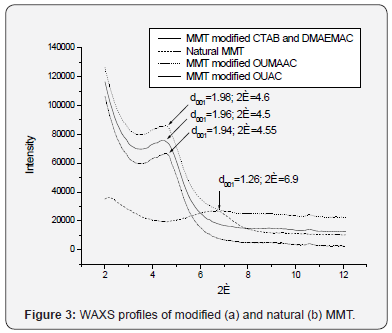
X-ray study of natural and modified MMT
WAXS results (Figure 3) has shown, that modified MMT has
a larger interlayer spacing d001 in comparison with the original
natural MMT (d001 = 1.26nm).
The distance between the MMT layers after modification
increases up to 0.68 - 72nm. It should be noted that the “unification” of inorganic cations in the middle of the layers
in case of Na-MMT reduces the interlayer distance d001 till
1.15nm. Thus, the effect of increasing of the interlayer distance
of MMT as a result of modification with organic compound can
be estimated as 0.79 - 0.83nm.
The increase in the interlayer distance of MMT (d001) after
the modification testifies to intercalation of organic modifier
into the interlayer space.

Preparation of nanocomposites based on PUA and modified ММТ
Formation of nanocomposites based on PUA and modified
MMT was carried out in a solution of organic solvent. In the case
of MMT, modified jointly with CTAB and DMAEMAC we used
toluene, in a case of MMT, modified with OUAC and OUMAAC we
us dimethylformamide (DMF).
PUA and MMT/M based nanocomposites were prepared in
toluene/DMF solution. Solution of toluene/DMF with calculated
amount of OUA and modified MTT was sonicated in a glass
container, then added to the reactor and stirred at 90 0C for five
hours. Then the photoiniciator Darokur-1173 in the amount of
2.5% per OUA weight was added to the reaction mixture which
was stirring for one hour. After stirring the resulting mixture in
toluene/DMF solution was poured from the reactor into a conical
flask with a stopper wherein the resulting mixture was settled.
Settled mixture was cast on glass Petri plates in equal
portions to produce the films of uniform thickness. The films
were formed by the gradual evaporation of the solvent under
normal conditions. The resulting films were irradiated with a UVlamp
for 30min at irradiation intensity of 8W/m2. Conversion of
methacrylate groups was determined by IR spectra: in 20min.
the band corresponding to methacrylate groups has disappeared.
Thus, by photoinitiated polymerization of OUA the films of
polymer matrix PUA and in situ nanocomposites PUA/MMT/M
with the content of modified mineral of 2.5wt% have been
obtained.
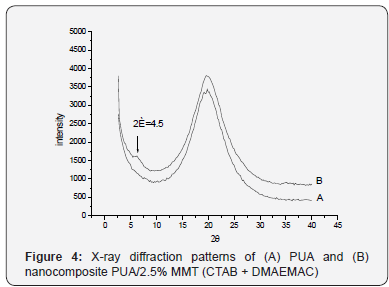
WAXS patterns of PUA/ММТ/M nanocomposite
It was found by WAXS method that exfoliation of nanofiller
in MMT modified with CTAB + DMAEMAC based nanocomposite
was not observed. Lack of exfoliation is illustrated by the
presence of absorption peak characteristic for the modified
MMT on the curve corresponding to the nanocomposite (Figure
4).


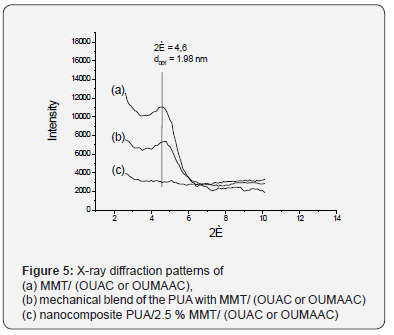
Unlike the first type of nanocomposite, the complete
exfoliation of the nanofiller was observed for nanocomposites
based on MMT modified with OUAC or OUMAAC. The absence
of the characteristic absorption peak of the MMT/ (OUAC
or OUMAAC) in nanocomposites’ WAXS patterns (Figure 5)
containing 2.5wt% of the nanofiller, testifies to the it complete
and systematic exfoliation in the PUA matrix. The absorption
peak 2Θ = 4.6 in the diffraction pattern of mechanical mixture
(b) is characteristic for the MMT/M, indicating that polymer
matrix by itself does not affect the character of MMT (OUAC or
OUMAAC) radiation absorption.
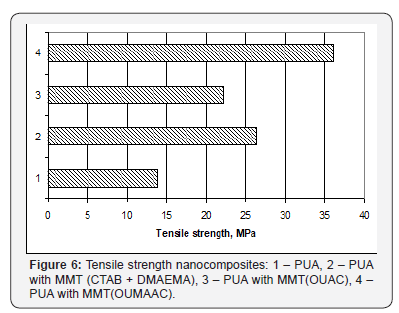
Physic-mechanical properties nanocomposites
Comparative analysis of the tensile strength of all obtained
nanocomposites with the same content of modified MTT
(2.5wt%) has shown a significant increase of tensile strength
relative to the original polymer matrix PUA (Figure 6).
It was found that nanocomposite with MMT (CTAB +
DMAEMA) has in 1.9 times higher strength than that of the
original PUA matrix. Increase of the strength of nanocomposite
with MMT (CTAB + DMAEMAC) may be the result of chemical
bonds formation between PUA and DMAEMAC on the MMT
surface.

Nanocomposite with MMT (OUAC) and nanocomposite with
MMT (OUMAAC) possess the strength in 1.6 and 2.6 times higher
than that of the original PUA matrix, correspondingly. Such
difference in the results is probably due to the fact that MMT
(OUAC) cannot form chemical bonds with PUA matrix.
Comparative analysis of the strength indices of all obtained
nanocomposites has clearly illustrated the strengthening effect
of reactive modifiers DMAEMAC and OUMAAC.
Nanocomposite with MMT (OUAC), despite the exfoliation
of nanofiller and the formation of hydrogen bonds with PUA
macromolecules, is inferior to nanocomposite with MMT (CTAB
+ DMAEMAC) for which the exfoliation of the nanofiller is not
typical.
Conclusion
Three methods of chemical modification of the layered
silicate surface have been developed.
a) New polyurethane acrylate nanocomposites based on
newly created modified MMTs with high performance have been
synthesized by in situ polymerization method.
b) The first modification method is based on using of
two different functional modifiers (organophilic and reactive),
the second method is based on modification with synthesized
by us compound which contains urethane groups, and the third
one in based on using of synthesized by us modifier containing
urethane and other reactive groups.
c) Developed polyurethane acrylate/organoclay
nanocomposites have shown a significant increase of strength
indices.
d) The complete exfoliation of the nanofiller in
nanocomposite based on MMT modified with OUAC or OUMAAC
has been confirmed by WAXS.
Developed methods of montmorillonite modification
are universal and can be applied to MMT of various origins
(deposits).
For more articles in Academic Journal of Polymer
Science please click on:
https://juniperpublishers.com/ajop/index.php
https://juniperpublishers.com/ajop/index.php


Comments
Post a Comment