Polymer-Based Approach in Ceramic Materials Processing for Energy Device Applications-Juniper Publishers
JUNIPER
PUBLISHERS- Academic Journal of Polymer Science
Abstract
Polymer-based approach such as sol-gel method is a
well-known method to produce ceramics materials with excellent
properties for a better performance of solid oxide fuel cell. The
properties of the materials are generally controlled by chemical agents
used in this method. The roles of the chemical agents including
chelating agent, polymerization or esterification agent and surfactant
are presented and briefly discussed in this mini review paper.
Keywords: Sol-gel method; Polymerization agent; Surfactant; Electrolyte; Anode; Cathode; Solid oxide fuel cell; MicrostructureAbbrevations:SOFCs: Solid Oxide Fuel Cells; SSR: Solid-State Reaction; WCMs: Wet Chemical Methods; CA: Citric Acid; EDTA: Ethylene Diamine Tetra-Acetic Acid; EG: Ethylene Glycol; TETA: Triethylenetetramine; PEG: Poly-Ethylene Glycol; PVA: Polyvinyl Alcohol; PVP: Polyvinyl Pyrrolidone; YSZ: Yttria-Stabilized Zirconia; AC: Activated Carbon
Introduction
High temperature perovskite-type oxide conductive
ceramics have attracted great attention worldwide due to the fact that
these materials have a great potential to be used as electrolyte and
cathode components in solid oxide fuel cells (SOFCs). SOFC is currently
deemed as one of the most promising future power generation devices due
to its high energy conversion efficiency, less/zero pollutant emission
and able to operate on various fuels. Two major concerns that limited
the performance of the current developed SOFC systems are low
electrolyte conductivity and high electrode polarization resistance
[1,2]. Controlling and modifying the microstructural properties of the
ceramics components of SOFC is a promising way to tackle the concerns
and could be achieved by selecting suitable ceramics processing routes
as they greatly affect the microstructure properties of the produced
ceramics materials [3]. Traditionally, a Simple Solid-State Reaction
(SSR) method is used to prepare the perovskite-type oxide ceramics
materials [4-7]. However, this method resulted in a poor microstructural
property of the produced powders due to high temperature of treatment
(> 1400 °C) and the produced powders are frequently contaminated
[8,9]. Hence, Wet Chemical Methods (WCMs) are introduced to overcome the
drawbacks of the SSR method. The WCMs are able to produce fine powders
with high purity and good homogeneity at lower
processing temperature than that of the SSR method [3,10]. One the most
popular WCMs is a sol-gel method. The preparation of materials through
this method is thoroughly discussed in the following section.
Sol-gel Method
A sol-gel method has been introduced in the 1800s to
produce inorganic ceramics and glass materials [11]. It is a process to
form oxide linkages via inorganic polymerization reaction. It starts
with a reaction between molecular precursor and solvent to form metal
organic complexes. The complexes will undergo polymerization process to
yield colloid or sol followed by hydrolysis to form a gel. Then, the gel
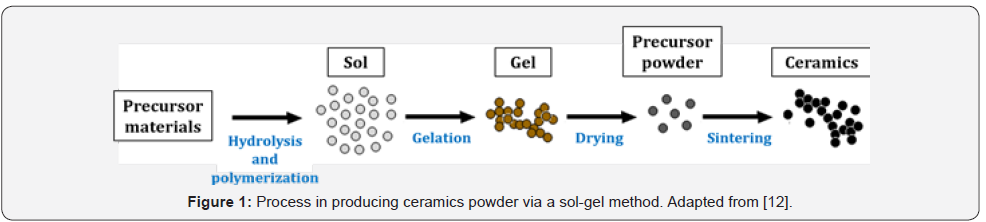
will turn into ceramics powder after drying and sintering processes
[12]. A simple illustration of the sol-gel processes is shown in Figure
1.
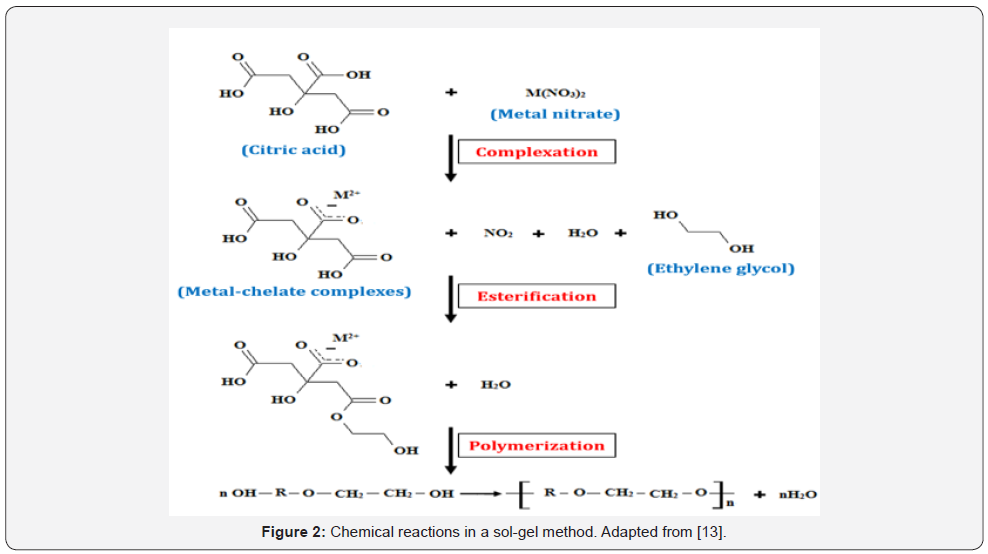
There are two basic chemical reactions involve in
sol-gel method which preserve the homogeneity of the metal salts
(precursor materials) in the solution into gel. The first one is the
complexation between metal ions and chelating agent such as Citric Acid
(CA) and Ethylenediaminetetra-Acetic Acid (EDTA) which provides a stable
metal-chelate in the solution by preserving atomic scale homogeneity.
Another one is polymerization of the complexes with polymerizing agent
or surfactant such as Ethylene Glycol (EG) which forms threedimensional
structures that hinder ion mobility and segregation.
A simple illustration of the chemical reactions which involves in
this method is shown in Figure 2 [13].
Roles of Chemical Agents
Generally, a chelating agent acts to bind all the metal cations
of the precursor materials to form a stable metal complexes in
a precursor materials solution with homogenous distribution
at atomic or molecular level. The addition of chelating agent is
able to control the rate of hydrolysis reaction, phase transition,
particle size and powder morphology [14]. CA and EDTA are
the most conventional chelating agents used to produce various
ceramics materials for SOFC application. They can be used
separately as a single (CA/EDTA) chelating agent [15-17] or
together as a combined (CA-EDTA) chelating agent [18-20]. A
combined chelating agent is better than a single chelating agent
because it is able to bind almost all metal cations and form more
stable complexes than a single chelating agent. Moreover, it is
less sensitive to pH value of the complexes solution and able
to reduce the temperature required for single phase powder
formation [21,22]. Besides, non-conventional chelating agents
such as Triethylenetetramine (TETA), glycolic acid, nitriloacetic
acid and tartaric acid have been also used to improve the
properties of BaCe0.54Zr0.36Y0.1O2.95 electrolyte material for
proton-conducting SOFC application [23].
Ethylene Glycol (EG) is a conventional solvent used in a solgel
method. It acts as polymerization agent or esterification agent
to produce cathode and electrolyte materials such La0.6Sr0.4CoO3-δ
[20], BaCe0.54Zr0.36Y0.1O2.95 [24], Sm1-xCaxFeO3 [25] and SrCox
[13] for SOFC applications. EG aids to form a stable polymer resin
of metal-chelate complexes. The formation of polymer resin
hinders the formation of particle agglomeration by forming rigid
network which controls the movement of metal cations in the
complex solution of precursor materials during heat treatment
process [26-28]. For better properties of the produced powder,
the amount of chelating agent and polymerization agent need to
be optimized and controlled.
In a modified sol-gel method, a surface-active agent or
simply known as surfactant is used to replace EG as a solvent.
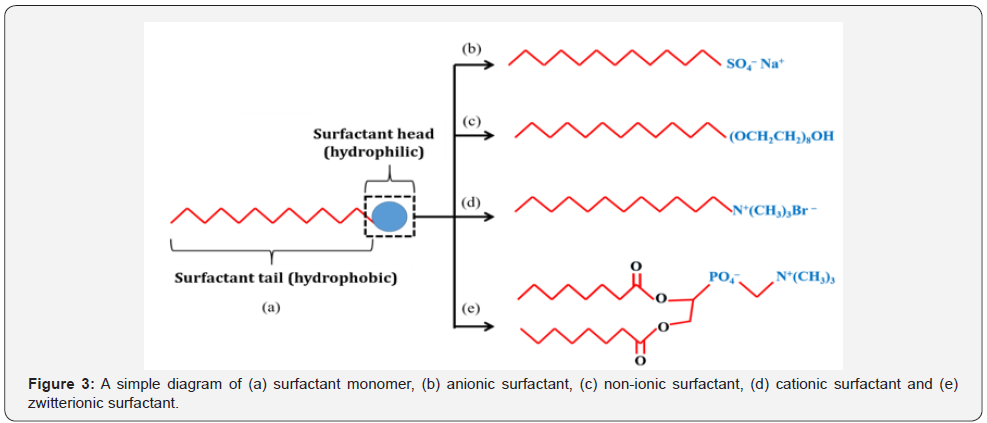
Surfactant is an amphiphilic compound consists of hydrophilic
polar group and hydrophobic non-polar group. The polar and non-polar groups are the head and tail of a surfactant monomer,
respectively. Surfactant is classified into three groups based
on the charge of the polar group which are ionic (anionic or
cationic), non-ionic and dipolar or zwitterionic. The non-polar
group is made up of long-chain hydrocarbon or siloxane chain
[29]. A simple diagram of a surfactant monomer is shown in
Figure 3.
A surfactant can increase miscibility, colloidal stabilization
and particle dispersion in a material with various components
because of its unique properties of self-assembly. These
properties help to reduce the tension of two or more components
in a solution system and change the properties of the surface
of the solution and increase the compatibility between the
particles with different properties in the solution [30,31].
In addition, a surfactant can control the shape and particle
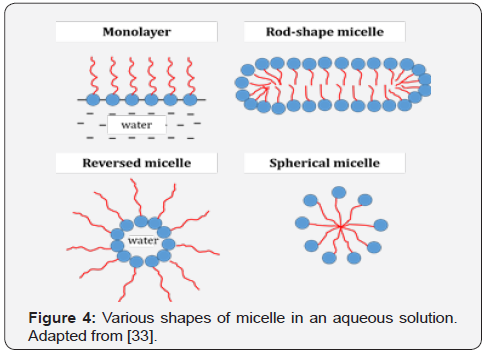
size of the produced ceramics materials. It forms a cluster of
thermodynamically stable supramolecular known as micelle
or microemulsion [32]. Micelle can form in various shapes in
an aqueous solution as shown in Figure 4. It is dependent on
temperature, surfactant concentration, surfactant composition
and pH [29]. The variation in the micelle shapes is one of the
factors that affects the particle shape and size of the produced
powder [24].
There are many surfactants which have been used to produce
ceramic materials for SOFC components via a sol-gel method [33].
Pluronic F127 (tri-block copolymer) and Triton-X-100 were used
to synthesis La0.58Sr0.4Fe0.8Co0.2O3-δ [34,35], and Poly-Ethylene
Glycol (PEG) was used to synthesize La0.6Sr0.4CoO3-δ cathode
materials. Polyvinyl Alcohol (PVA) and Polyvinyl Pyrrolidone
(PVP) were used to synthesize La0.8Sr0.2MnO3-δ cathode material
[36]. All of these surfactants helped to reduce the particle size
from micro to nano, increase the surface area and homogeneity
of the produced powders. The same results were also reported
for the synthesis of electrolyte materials of Yttria-Stabilized
Zirconia (YSZ) [37], Ce0.9Gd0.1O1.95 [38], Ce0.8Sm0.2O2-δ [39], and
BaCe0.54Zr0.36Y0.1O2.95 [24], using various surfactants. Additionally,
surfactant also helps to reduce the temperature required to
produce single phase powder and high-density pellet [38,40].
In addition, Activated Carbon (AC) has been introduced
as a dispersing agent in a modified sol-gel method to produce
perovskite-type oxide conductive ceramics for SOFC application.
The use of AC as a dispersing agent is quite a new invention
in sol-gel method. AC, which is a treated form of carbon with
high degree of microporosity, surface area (300-2000m2g-1)
and well adsorption ability, is used to replace the conventional
solvent or surfactant in the conventional sol-gel method. Like
the other chemical agents, AC is also responsible for controlling
the nucleation process, phase development and particle growth
during the synthesis process. However, it has different reaction
mechanism as compared with the other chemical agents. AC traps
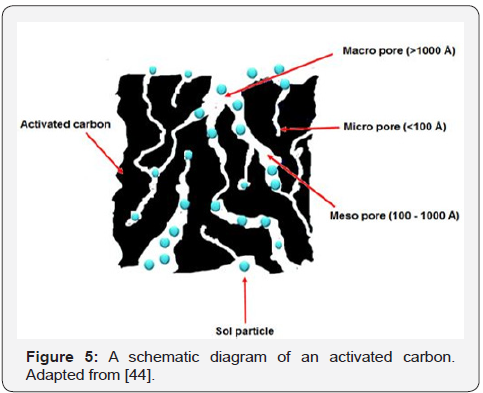
the metal complexes in precursor solution in its highly porous
microstructure through van der Waals attraction, creating
well-dispersed particles of the produced ceramic materials
after calcination process (Figure 5). Its potential in modifying
microstructure of perovskite-type ceramic materials with good
electrochemical performance particularly for SOFC application
have been reported by Ismail et al. [41] and Abdul S et al. [42,43].
The improved properties of the produced ceramics materials
by sol-gel method are very important for better electrochemical
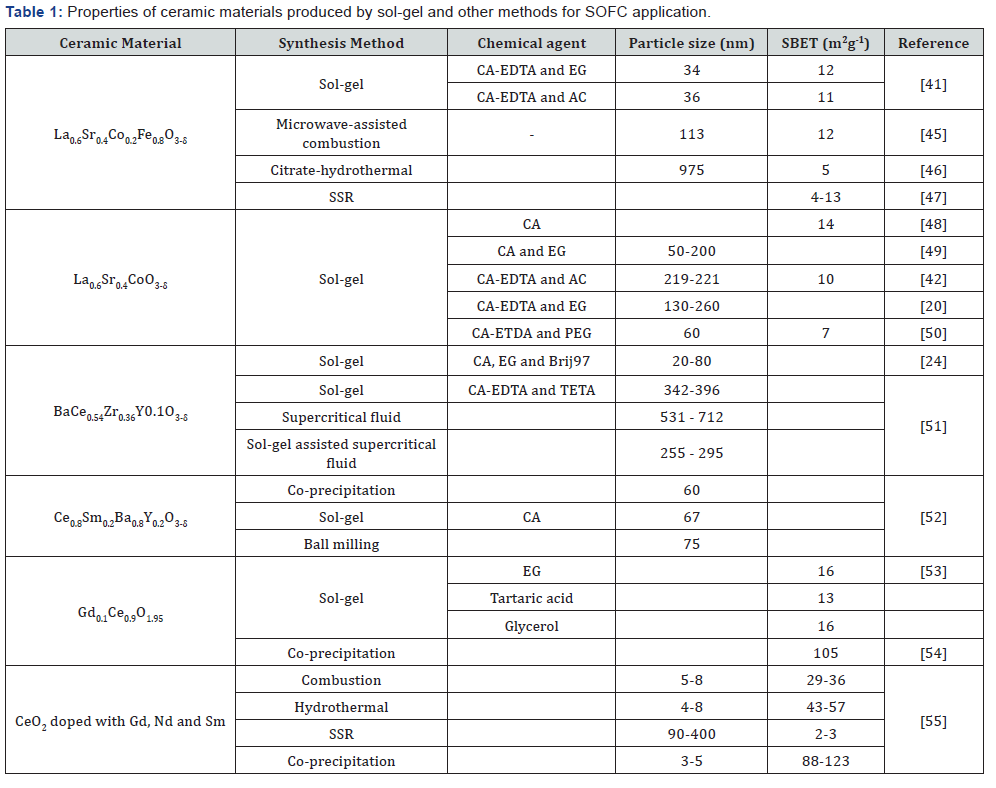
performance of the SOFC components [44-48]. The properties
i.e. particle size and BET specific surface area (SBET) of some
common ceramic materials for SOFC application produced by
different chemical agents via sol-gel method and other synthesis
methods are tabulated in Table 1. It can be seen that the
properties of the produced ceramic materials are different. It is
due to the different chemical agents used in the sol-gel method
and the different in the method used to produce the ceramic
materials [49-52]. The discrepancy in the mentioned properties
might also be contributed by the different in the heat treatment
process and synthesis parameters applied to obtain a single
perovskite phase of the ceramic materials [53-55].
Conclusion
A sol-gel method is regarded as a promising synthesis route
to produce better properties of ceramics materials for SOFC
electrolyte and cathode components. The chemical agents
used in this method have been proved to significantly affect
the properties of the produced materials such as particle size
and SBET. Given that research on the sol-gel method has been continuously improving with time, many other materials
can be used as chemical agents. Additional studies of basic
understanding on how the chemical agents work and what are
the condition they work at the best, including composition,
pH, concentration and processing temperatures much be
investigated in detail.
Acknowledgement
The authors would like to acknowledge the Research
University grant (DIP-2016-005) provided by Universiti
Kebangsaan Malaysia. Abdullah Abdul Samat thankfully
acknowledges the Ministry of Higher Education of Malaysia and
Universiti Malaysia Perlis for the SLAB/SLAI PhD scholarship.
For more articles in Academic Journal of Polymer
Science please click on:
https://juniperpublishers.com/ajop/index.php
https://juniperpublishers.com/ajop/index.php


Comments
Post a Comment