Microdroplet Chemistry: Difference of Organic Reactions between Bulk Solution and Aqueous Microdroplets-Juniper Publishers
Authored
by Inho Nam

Abstract
Recent applications of microdroplet reactions are
noted as reaction acceleration in confined environments and possible
future scale-up synthesis compare to that of bulk phase reactions.
Microdroplet reactions overcome the thermodynamic and kinetic challenges
in an aqueous solution. Due to the microdroplet’s distinctive surface
environment, the chemistry in Microdroplets significantly differs from
that in the bulk solution.
Keywords: Microdroplet; Noncovalent complexations; Electrostatic potential; Organic reactions
Introduction
Remarkable findings intrigued people in different
fields by showing that extremely slow bulk phase reactions can be
accelerated dramatically in aqueous microdroplets [1,2]. Not only the
rate of the reaction but also the yields of reaction increased due to
negative free-energy change which is lower than the value in the bulk
phase. Recent experiments have been done by several groups and they
validated how microdroplet reactions enable to accelerate organic
reactions such as addition reactions [3], condensation reactions [4,5],
elimination reactions [2], substitution reactions [2], redox reactions
[6],
rearrangement reactions [7], and noncovalent complexations [8]. In this
mini-review, we show the recent progress about the kinetic and
thermodynamic change of organic reactions in
aqueous microdroplets, as names as microdroplet chemistry, and its
limitation.
To address how reactions in water microdroplets can
significantly differ from those in the bulk phase, many experiments have
been done and gave clues for the understanding (Table 1). In specific,
the reduction of 2,6-dichlorophenolindophenol by the microdroplet fusion
method is accelerated by a magnitude of 103 [6]. Base-catalyzed
Claisen-Schmidt condensation of 1-indanone with electro spray ionization
[ESI] is accelerated by a magnitude of 104 [4]. And the
Pomeranz-Fritsch synthesis of isoquinoline, which is done by using ESI,
is also accelerated by the order of 106 [7]. These reactions
substantiate an increased rate in the microdroplet reaction by many
orders of magnitude compared to the extremely slow kinetics in the bulk
phase [1,2].

Within the distinctive environment of water microdroplets
with different thermodynamic and kinetic properties compared
to the bulk phase, an intriguingly plausible biochemical reaction
mechanism in aqueous microdroplets were also reported, which
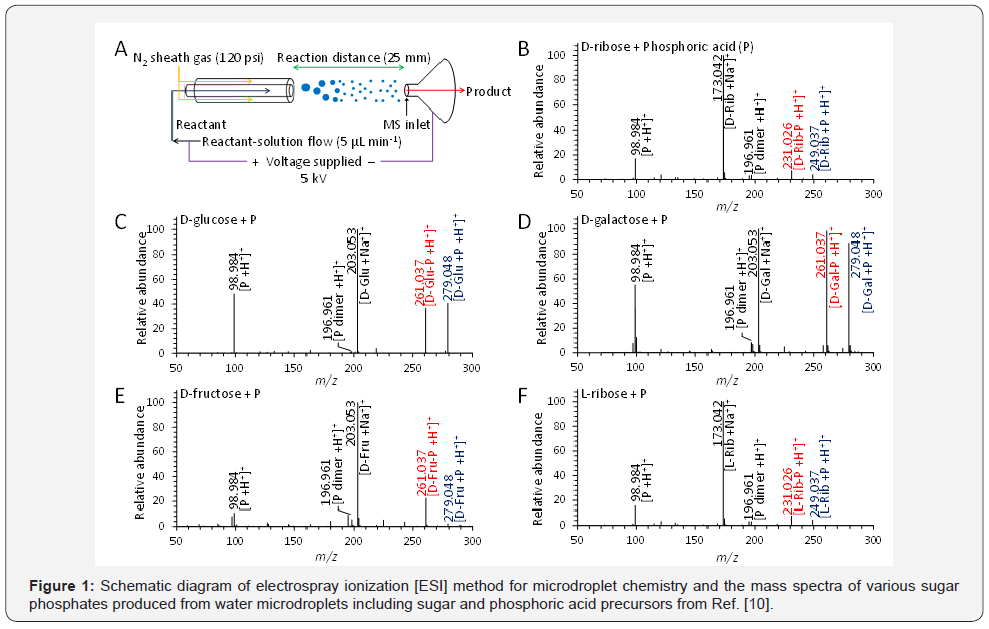
might occur prebiotically in the absence of enzymes [9,10]. The
abiotic production of sugar phosphates and ribonucleosides
naturally does not occur in bulk aqueous solution, because of
their thermodynamic hurdle [11]. Within a bulk phase, both
phosphorylation and ribosylation of ribose, phosphate and
nucleobases are extremely unfavorable, due to condensation of
reagents accompanied by the elimination of water [12]. These
reactions in bulk phase have an extremely low equilibrium
constant [Keq] and a positive free-energy change, which leads to
reverse process of hydrolysis of the biopolymer. On the contrary,
the reaction in microdroplet provides a negative free-energy
change. It substantiates a possible route for the prebiotically
plausible formation of sugar phosphates and ribonucleo sides
in microdroplets as shown in Figure 1. In the crowded interior
of the microdroplets, longer range attractive forces can provide
organization of molecules and decrease the organic reaction’s
Gibbs free energy [13]. This leads favorable change in free
energy and acceleration in reaction rate along with high yields.
In consequence, although there is lack of evidence, the unique
reaction environment by the surface of microdroplets might
provide the clue of prebiotic chemical interaction which is
significantly related to the possible routes of the origin of life
[14]. Because, water-air interfaces on oceans, lakes, aerosols,
and cloud and fog droplets can provide reaction environments
for the abiotic organic reactions [15,16].

The organic reactions in water microdroplets [Microdroplet
chemistry] are related to the concentration change of reactants
in surface of microdroplets and the distinctive trait of the
surface [1,2]. First, the concentration of reactants increases by
the evaporation of a solvent on the surface of microdroplets,
which makes a reaction acceleration [2]. In chemical reaction,
Keq, is a value that never changes. To maintain that value, as the
amount of reactants increases, the amount of the products must
increase as well. Second, there is an orientation of reactants by
the electrostatic potential on the surface of microdroplets, which
brings about a decrease of an entropic hurdle in the chemical
reaction [17]. At the interface, the characteristics of liqiudare
different from in bulk solution. It means that the entropy of
water in bulk is higher than at water-air interface and that there
is very little chance that the water will react and form products.
But, it is different when dealing with the water molecules at
the air-liquid interface [9,10]. Near the interface, the water
molecules organize and get anisotropic structures. This leads
water molecules to gain charges and form an electric field, which
affects the reaction rate [18]. That can make multiple parameters
that affect the chemical reaction in the microdroplet such as pH,
surface charge, reagent confinement, desolvation, contact ion
pairing, large electrostatic pressure and molecular orientation
on the droplet surface [9,10].
The above observations seem too common related to the
kinetic and thermodynamic changes of organic reactions in
water microdroplets. The superfast and thermodynamically
changed organic reactions are maybe possible to open new
chapter of chemistry [2]. At this chapter, we need to more
precisely consider if there are some limitations in microdroplet
chemistry. As shown before, many researches on acid-or basedcatalyzed
reactions or the reactants containing polar functional
groups proved that the reactions in the microdroplet are
accelerated or thermodynamically changed [2-10]. However,
there is little information about nonpolar compounds reaction
in microdroplets. As a model of non polar organic reaction, the
intra molecular Diels-Alder reaction of 3,5-hexadienyl acrylate
ester was conducted recently [1]. The bulk phase, When the
reaction in the bulk phase was performed with a catalyst of
indium [III] triflate, it was reported that the reactants changed
to Diels-Alder product at 70oC.When the reaction is conducted in
microdroplets, the expected product is not observed, buthexa-
3,5-dien-1-ol was detected. It notes that the interface factor
negligibly affects the nonpolar organic reaction because there is
little chance of the special polar surface of microdroplets favor
nonpolar reactive reagents [1].
Conclusion
We introduce the recent success of microdroplet chemistry
in view of the acceleration and thermodynamic preference
change of general organic reactions. The reaction mechanisms
are not completely understood, there are two main factors for
the favorable change of the kinetics and thermodynamics of
reactions in microdroplets. Concentration is one factor that
attributes to the acceleration of the reaction rate in confined
aqueous environments [2]. Because of the surface area of the
droplets increases, the area in which droplets can evaporate
increases as well compared to that of the bulk solution. This
leads to an increase in the concentration of the reactants. To
balance the equilibrium, the products increase as the reactants
increase, which leads to a higher amount of yields. Second,
the natural high surface to volume ratio of microdroplets can
generate unique surface characters at the air-water interface [1-
8]. As molecules in water droplets approach closer to interface
between water and air, their organized water-air environment
leads electric field to alter pH, surface charge, and orientation
of reagents at surfaces [19,20]. Even though there is a limitation
that water microdroplet chemistry is not favorable to nonpolar
interaction, the water microdroplet chemistry can lead to facile
organic synthesis technique and give the precise knowledge
of chemistry in confined environment including biochemical
processes in a cell nature [14].
Acknowledgment
This research was supported by a research grant from Seoul
Women’s University (2018).
For more
details Academic
Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
To read more…Full Text in Juniper Publishers click on https://juniperpublishers.com/ajop/AJOP.MS.ID.555551.php


Comments
Post a Comment