Energy Potential of Natural, Synthetic Polymers and Waste Materials - A Review-Juniper Publishers
Authored by Michael Ioelovich
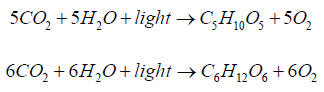
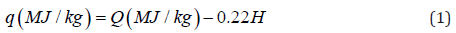



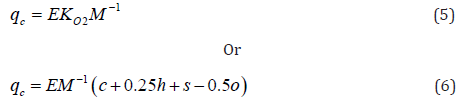




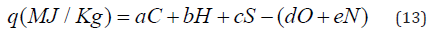
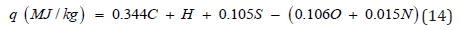









Abstract
In this paper, the energy potential of natural
(polysaccharides, lignin, etc.), and synthetic polymers (polyolefins,
etc.), polymer materials (plant biomass, plastics and their solid
waste), as well as combustible liquids and gases derived from them has
been studied. To determine calorific values of solid, liquid and gaseous
substances, various experimental and calculation methods were used. For
this purpose, improved equations based on chemical structure and
elemental analysis was proposed. It was shown that the conversion of
solid materials into liquids or gases reduces the yield of thermal
energy. Therefore for the production of thermal energy, it is more
profitable to burn the solid biomass or plastic than combustible liquids
and gases, derived from them. Studies have shown that lipids and lignin
increase, whereas moisture and ash reduce the calorific value of
biomass. The calorific value of most synthetic polymers and plastics is
higher than that of biomass samples, but resources of waste plastics are
about 10 times lower than these of biomass. The use of compacted
mixture of biomass and plastic waste enables to obtain solid fuels with
unique properties, such as increased calorific value and energy density,
as well as reduced emission of carbon dioxide. Considering the total
amount of biomass and plastics waste destined for combustion, it was
calculated that current annual energy potential of the waste materials
is about 145 EJ. The increase the share of biomass in the production of
alternative energy can simultaneously contribute to reducing in
greenhouse gas emission and improving the ecological state of the
environment.
Keywords:
Plant polymers; Biomass; Synthetic polymers; Plastics; Solid waste;
Solid fuel; Liquid fuel; Gaseous fuel; Calorific value; Energy density;
Energetic potential
Introduction
The supply of sustainable energy is the basis of
modern civilization. Nowadays, the main energy sources are fossil fuels,
coal, petroleum and natural gas, which cover up to 80% of global energy
consumption [1]. However, the use of fossil fuels causes the emission
of large volumes of greenhouse gas - carbon dioxide, in the amount of
1600-1800m3 per ton of fuel. The further intensive energy consumption
can leads to irrevocable depletion of reserves of these fuels. Therefore
in recent years a considerable attention has been paid to plant biomass
as inexpensive and inexhaustible source of renewable energy [2].
The term “biomass” means here a variety of plant
materials, as well as their residues and wastes [3]. Total resources of
such biomass reach 1.5 trillion tons and increase by 200-250 billion
tons annually as a result of the photo- and biosynthesis [4]. The main
structural constituents of plant biomass are three natural polymers, and
namely cellulose, hemicelluloses, and lignin. Being the most abundant
organic matter on Earth, cellulose forms the skeleton of cell walls of
various plants. The content of cellulose in herbaceous plants is 30 to
40%, in woods 45 to 50%, in best plants (flax, ramie, jute, etc.) 60 to
70%, and in cotton fibers upwards of 90% [5].
Cellulose is a natural semi crystalline
polysaccharide having long linear chains consisting of repeat
anhydroglucose units in a “chair” conformation [6]. The linear
macromolecules joined by hydrogen bonds form super molecular structure
of cellulose that consists of thread-like elementary nanofibrils and
their bundles called micro fibrils. Within the elementary fibrils,
ordered crystallites and disordered non-crystalline domains (DD) are
present. During the process of isolation and modification of cellulose,
the cleavage of glycosidic bonds in DD occurs, which leads to partial
depolymerization of this polysaccharide. Hemicelluloses are
non-cellulose polysaccharides comprising of pentosans and hexosans [1].
The content of these components in various plants is 20 to 40%.
Hemicelluloses are hydrophilic amorphous heteropolymers. The chemical
structure of hemicelluloses consists of chains of a variety of
acetylated links of pentoses or hexoses and backbones [7]. In the plant
cells, hemicelluloses are binders between fibrils of cellulose and
lignin.
Lignin is a rigid, aromatic, amorphous and
hydrophobic polymer that is stable to some chemical reagents and
cellulolytic enzymes [7]. The content of lignin in plant biomass ranges
from 10% for corn cob or rice straw to 48% for olive husk [8,9]. Lignin
is a complex polymer of phenylpropane units, which are cross-linked
to each other with a variety of different chemical bonds [10]. In the
lignified plant biomass, one portion of lignin is localized between
the cells, while another portion of lignin is localized inside the cell
wall in the form of thin hydrophobic nano-layers. The cellulose
nanofibrils inside the plant cell wall are separated from each
other by means of the thin layers of amorphous hemicelluloses,
whereas fibrillar bundles consisting of the nanofibrils of cellulose
and hemicelluloses are surrounded with hydrophobic nano-layers
of lignin, which protect the hydrophilic polysaccharides against
biological and chemical attack [11].
Polymeric constituents of biomass are formed in nature by
photosynthesis absorbing the solar energy. At first, monomeric
sugars are synthesized in chlorophyll pigment of plant leaves from
carbon dioxide and water absorbing quanta of red and blue-violet
light [12,13]. Photosynthesis is a complex, multistage process that
is proceeding in two phases - light and the dark. The light phase
occurs in the presence of light in the thylakoid membranes of
chlorophyll, with the participation of electron transfer proteins,
specific carrier NADP and ATP-synthase, the result of which is
photolysis of water molecules, binding of formed hydrogen by
NADP and release of oxygen. The dark phase proceeds without
light with use of NADP • 2 H complex and carbon dioxide and their
transformation to monomeric sugar with the participation of ATP.
Summary process of photosynthesis of monomeric 5 C - and 6 C
-sugars can be described as follows:
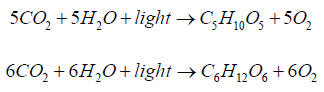
At biosynthesis of cellulose and starch, glucose is used as
C6-monomer in polycondensation reaction with formation of
long polymeric chains. Cellulose of plants is formed on the
surface of plasma membranes of living cells using a terminal
enzymatic complex shaped as a nano-rosette with a hole in the
middle [14,15]. At biosynthesis of hemicelluloses, hexosans
and pentosans, photo-synthesized hexoses (e.g. mannose) and
pentoses (e.g. xylose) participate in polycondensation reactions.
The photo-synthesized sugars are probably the precursors of
lignin biosynthesis in the plant cell walls [16,17]. In the process
of biochemical reactions these sugars form a series of aromatic
acids, aldehydes and alcohols, which are then polymerized into
lignin.
In fact, biomass can be considered an accumulator of solar
energy captured during photosynthesis. To generate the heat
energy the plant biomass is burned, resulting in the release of
accumulated solar energy. Various biomasses and plant-based
materials can be used as solid fuels such as wood (e.g. firewood,
pellets), herbaceous plants (e.g. Miscanthus, Switch grass,
Bermuda grass, etc.), forest residues (e.g. sawdust, twigs, shrubs,
etc.), residues of agricultural plants (e.g. stalks, husks, cobs, etc.),
residues and wastes of textile, pulp, paper and cardboard, as well
as algae biomass. Energy is also obtained by burning of refuse,
containing waste paper, cardboard, textile, wood and other plant
waste. In many countries, these bio-resources account for over
90% of household energy consumption [18].
A specific feature of plant biomass is that this solid biofuel is
neutral for emission of carbon dioxide, since its burning produces
the same amount of this green house gas as it was absorbed from
the atmosphere during biosynthesis. Furthermore, the biomass
can be used also as feedstock for production of liquid and gaseous
biofuels. Currently, plant biomass is the fourth largest energy
source after fossil fuels [19]. Moreover, it is the largest source of
renewable and alternative energy sources, far ahead of solar, wind
and nuclear power stations, as well as hydroelectric and some
other power stations [20,21].
An additional energy source can be waste of synthetic
polymers and plastics. At present, polymeric materials are widely
used for the production of packaging materials, consumer goods
and diverse industrial products. Every year in the world about
250-300 million tons of plastics are produced [22]. However after
usage, these biostable polymer products are thrown away and
pollute the environment. A huge problem is also the pollution of
water bodies and oceans with polymer debris [23]. The cardinal
way of eliminating biostable plastic debris is burning, which
allows also generating an additional thermal energy.
Thereby, the main purpose of this review was to evaluate the
energy potential of natural and synthetic polymers and materials
such as plant biomass, plastics and their solid waste, as well as
combustible liquids and gases derived from them, in order to use
them in energy production
Thermochemistry of Fuels
The specific heat energy or calorific value of solid fuels or
organic matters can be expressed by higher heating value (HHV) and
lower heating value (LHV) also called net combustion heat (NCH).
The HHV has the same meaning as the enthalpy of combustion,
which assumes a return of temperature to the standard value; in
this case the water vapor produced by combustion is condensed to
a liquid, hence yielding its condensation heat [24-26]. As opposed
to HHV, the LHV or NCH assumes that water remains in vapor
state at the end of combustion process [27-29]. Thus, the LHV or
NCH characterizes the actual combustion process. The following
notations were used to simplify the symbols: HHV=Q and LHV
(NCH) =q. These characteristics of calorific value are connected to
each other by the relationship [30]:
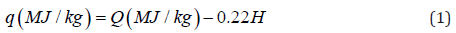
Where,
H is percentage of hydrogen in a sample
To study the calorific value of solid fuels or organic
matters various methods can be used. The first is experimental
determination of HHV, which is performed by combustion of organic sample in a special device, bomb calorimeter [31-34].
The calorimetric experiment can be briefly described, as follows.
The small sample (1g) is put into special container of calorimetric
bomb, which is filled with moist oxygen under increased pressure.
The bomb is closed and put into isothermal water calorimeter.
The system is thermo stated at 298.15K to achieve an equilibrium
state. After ignition of the sample, the temperature rise ( Δ T) is
measured. The higher heat of combustion is calculated from
equation:

where m is mass of the dry sample , C is value of energy
equivalent of the bomb calorimeter determined by burning of
standard benzoic acid; e1 and e2 is correction for ignition energy
and possible formation and dissolutions of acids (sulphuric
or/and nitric) from trace S or/and N elements of the sample,
respectively.
However, the bomb calorimeter is a complex, expensive
and not always available device. In addition, the measurement
of calorific value is lengthy and requires multiple repetitions to
obtain a reliable result.
Other method is based on Hess’s law, according to which the
combustion enthalpy at standard conditions can be calculated
from difference between formation enthalpies of combustion
products ( Δf Hpi ) and formation enthalpy of the combustible
matter ( Δf Ho ):

Wherein i is number of moles of various products of
combustion, i.e. CO2, H2O, SO2, etc
Consider, for example, burning process of cellulose:
C6 H10 O2 + 6O2 →6CO2 + 5H2 O
It can be found from thermodynamic handbook that
Δf Hp co2 = −393.51 KJ mol , Δf Hp H2o = − 285.83KJ mol
and Δf Ho2 = 0. Standard formation enthalpy of cellulose
is, on average: Δf Ho = − 965KJ mol [34,35]. Then, the
combustion enthalpy of cellulose can be calculated by eq. (2):
Δc H = (6X 393.51) -(5 X285.83) -965=- 2825( KJ/ mol)
. Thus, HHV of cellulose sample:
Q ≈ −Δc H = 2825KJ /mol17.4 MJ kg , and q =16.1MJ / KG .
Third method is based on the combustion process of organic
matter:
Cc Hh Oo Ss Nn+Ko2 O2 →cCO2 + (h/2 )H2 O + sSO2+(n/2)N2
Where KO2 is consumption of oxygen (moles per 1 mol of
the matter); c, h, o, s, n is number of the corresponding atoms
in molecule of low-molecular substance or in repeating unit of
polymer.
As it is follows from the combustion reaction,

According to hypothesis proposed in [36,37], a net combustion
heat (NCH) of organic matter is directly proportional to oxygen
consumption, i.e.:
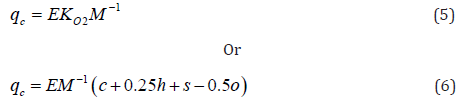
Where E ≈ 418KJ / mole 2 O is energetic parameter; M is
molecular weight of low-molecular substance or repeating unit
of polymer
By finding the KO2
value or
number of the atoms c, h, o and s
from combustion reaction, it can easily to calculate the net heating
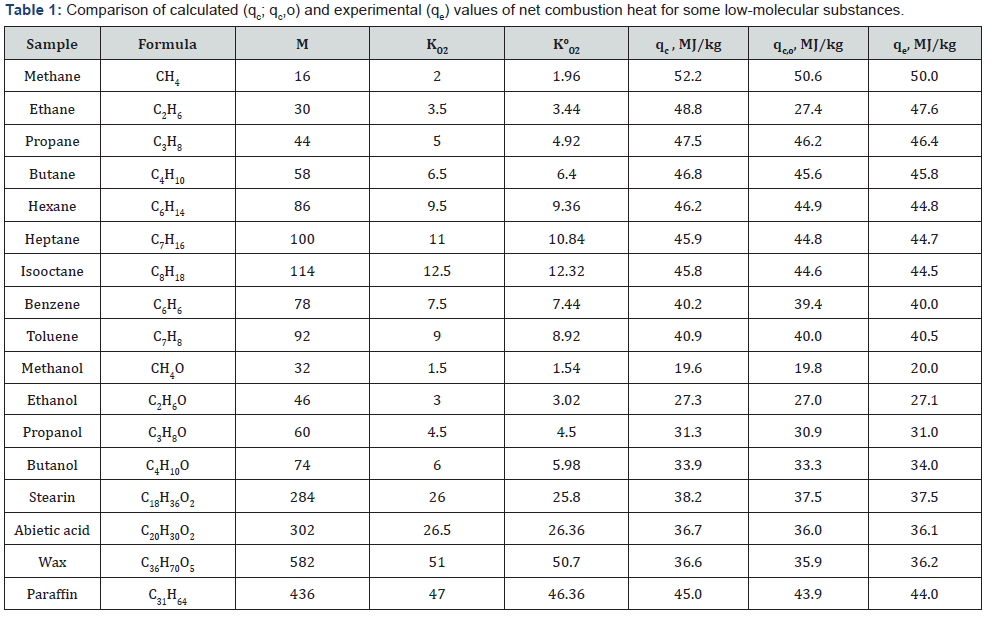
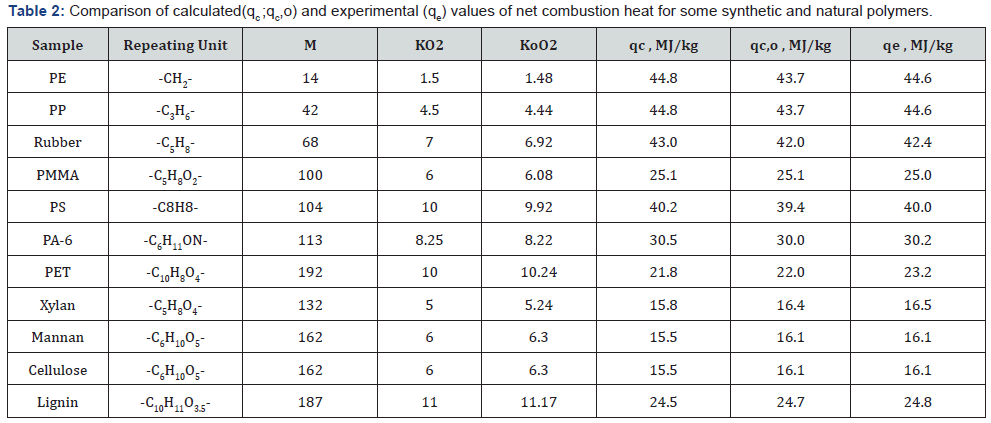
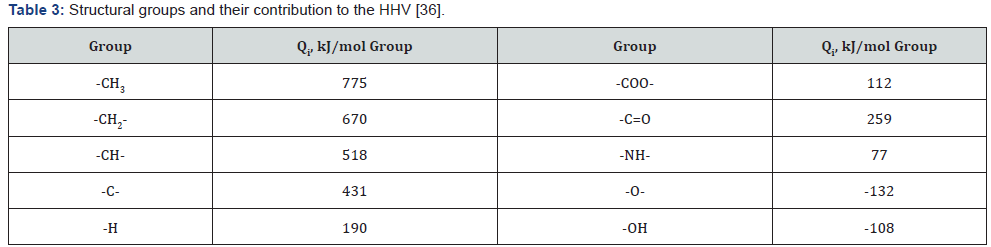
value of organic matter by eq. (4) or (5).The results showed that
the calculated values differ from experimental values of NCH
within ±6%. (Table 1 & 2).


In the case when it is necessary to find the HHV, the following
equation can be used:

To reduce the divergence between the calculated and
experimental values, several corrections were introduced in eq.
(4), (5) and (6). As a result, the following improved equations
were derived [38]:

Where E° = 413kJ/mole 2 O is corrected energetic parameter;
KO2° is corrected parameter of oxygen consumption.
The improved equations permit to reduce the discrepancy
between calculated and experimental values to ±1% (Table 1 &
2).
In addition, it is necessary to mention a few correlation
methods. One from them is based on the contribution of structural
groups to the HHV [36] (Table 3). HHV of the sample was calculated
by the equation:

Then, the NCH of the sample can be calculated from eq. (1)
or (12).


The verification showed that the method of contribution of
structural groups makes it possible to predict the heating value of
various organic substances and polymers with accuracy of ±5%.
Other correlation methods are based on the determination of
percentage of C, H, O, S and N atoms in molecule of low-molecular
substance or in repeating unit of polymer:
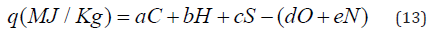
Where a, b, c, d, e – coefficients; C, H, O, S and N are percentage
of corresponds atoms calculating from formula of the organic
substance.
In the known equation of Mendeleev [39,40] a=0.339, b=1.035,
c=0.109, d=0.109 and e=0.015. Parikh et al. [41] proposed other
coefficients of eq. (12): a=0.349, b=0.960, c=0.101, d=0.103 and
e=0.015. Study have shown that the equation of Mendeleev gives
depressed values (-5%), whereas the equation of Parikh gives
overestimated results (+5%) in comparison with experimental
values of NCH. To increase the accuracy of calculations the
corrected coefficients were proposed [42]: a=0.344, b=1.000,
c=0.105, d=0.106 and e=0.015.
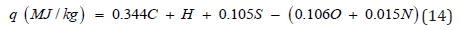
As a result, the difference between calculated and experimental
values can be reduced to ±1% (Table 4).

Thus, the best results give calculations by the equations (10)
and (14).
Energy Potential of Solid Polymer Materials and their Waste
As is known, about 200-250 billion tons of biomass are
created annually as a result of photosynthesis. Since biomass uses
only 1% of the incident solar irradiation, then total amount of
solar energy needed to create 200-250 billion tons of biomass can
be estimated at 300-400ZJ, whereas amount of accumulated solar
energy at 3000-4000EJ.
Most of the created biomass amount, 90%, is decomposed in
nature, and about 45-50% of the remaining biomass (wood, bast
plants, cotton, cereals, technical crops, etc.) are used in industry
and agriculture. Thus, the amount of unused biomass is about 10-
15 billion tons, which can be used as a source of alternative energy
[1,2].
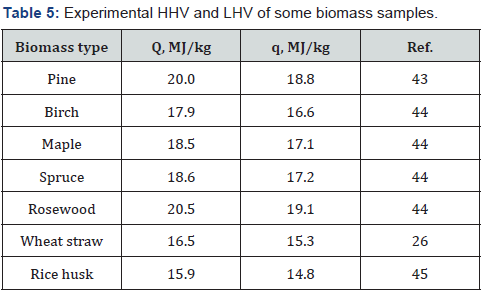
The use of biomass as a solid fuel requires knowledge of its
specific thermal energy, called also calorific value. Therefore,
numerous attempts have been made to determine the calorific
value of various types of biomass. Some of these studies have been
performed with the use of precise calorimetric technique [43-45]
(Table 5). The problem is that this technique is expensive and not
always available; besides, direct measurements are complex and
long. For this reason diverse express methods were developed
to calculate the calorific value of various plant biomass. These
methods are based on preliminary elemental and proximate
analysis and well as determination of chemical composition,
study structural characteristics and various properties of biomass
samples [46-50].

In contrast to plant polymers, the elemental composition of
such complex composite as biomass cannot be calculated from
chemical formula. Therefore, various experimental techniques
(e.g. gas analyzer, etc.) are applied to determine the percentage
of various elements in the plant materials. Proximate analysis of
biomass allows determining the content of ash, volatile matter
(VM), fixed carbon (FC). Standard methods of chemical analysis
are known to determine percentage of cellulose, hemicelluloses, lignin and some other components, such as starch, pectin,
proteins, extractives, etc. For example, the content of cellulose and
hemicelluloses in biomass can be determined using the standard
method NREL LAP-002, acid-insoluble lignin using the NREL LAP-
003, acid-soluble lignin using the NREL LAP-004, ash using the
standard method NREL LAP-005 and extractives using the NREL
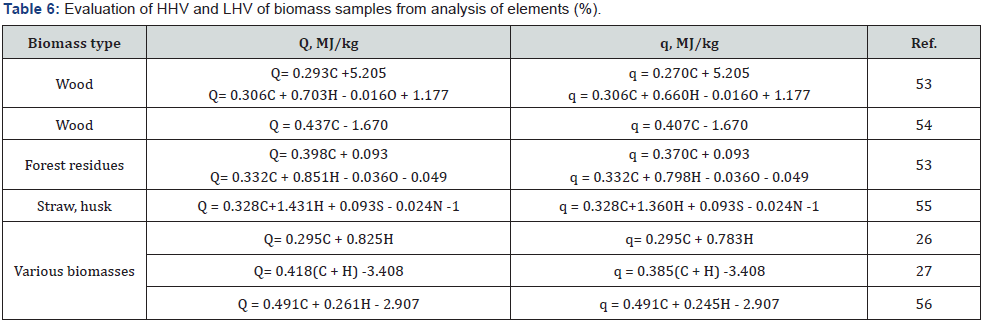
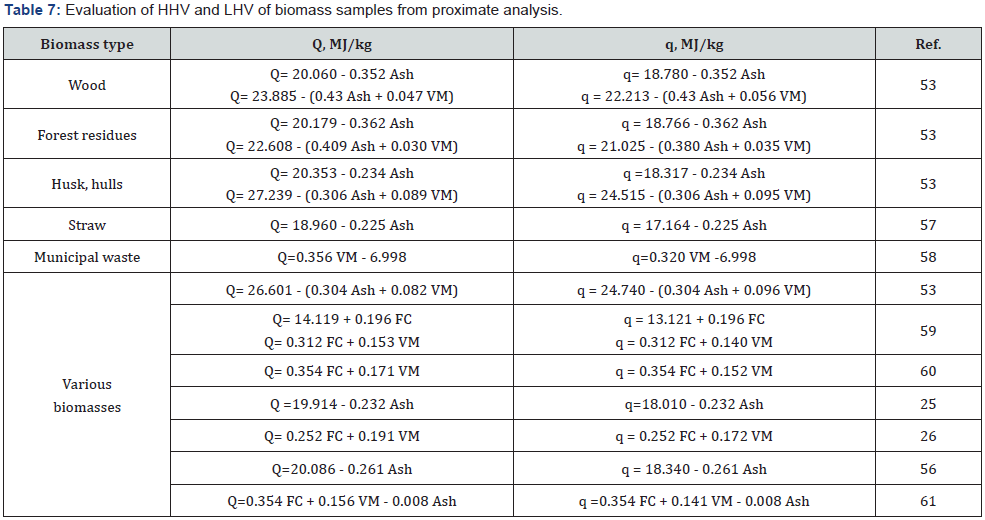
LAP-010 [51,52]. Some correlation equations for estimating the
HHV and LHV of different biomass types are presented in (Table
6 & 7).


The study of calculation methods based on proximate analysis
showed that the equations containing percentage of Ash, VM and
FC are insufficiently reliable, because the determination error
of these parameters is quite high. More reliable results can be
obtained using the equations based on the precise elemental
analysis of a specific type of biomass. For example, equation of
Tillman [54] is most suitable for calculating the heating value of
wood samples, whereas equation of Grabovsky & Bain [55,62] is
most suitable for estimating the heating value of straw biomass.
Calculations by means of “universal” equations averaging the
results of various biomasses show a higher discrepancy with
experiments than specific equations. Since an unlimited number
of different types of biomass exists, an unlimited number of
correlation equations is required to calculate the heating value. It
is clear that such task is not feasible.
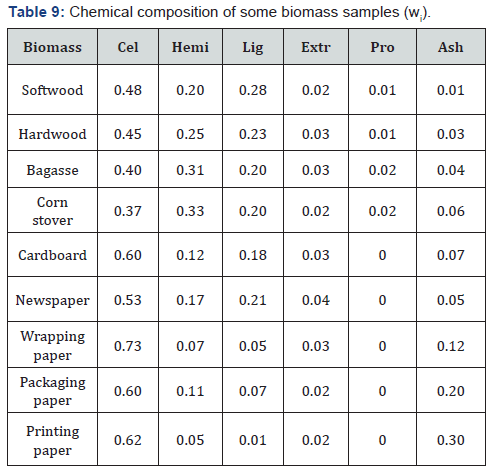
For this reason, another approach is proposed [63]. It is
known that any plant biomass contains three natural biopolymers,
cellulose, hemicelluloses and lignin, as well as extractives and
some other substances. Thus, to evaluate the heating value of the
biomass sample it is sufficient to determine the heating value of
limited number of individual components and their content in the
biomass, using the following equations:


where Qi and qi
are HHV and LHV (NCH)of individual
components in the biomass, which can be determined
experimentally or calculated using eq. (7), (10) or (14) (Table
8); wi is mass fraction of the corresponding components in dry
biomass sample (Table 9), which is determined by standard
methods of chemical analysis [51,52].


Note: Cel is Cellulose; Hemi is Hemicelluloses; Lig is Lignin;
Extr is Extractives; Pro is Proteins
Using eq. (14) and (15), HHV and LHV of biomass samples
can be easily calculated (Table 10).The results showed that this
approach provides to calculate the heating values, which are close
to experimental values.

Note: MP is mixture of NP, WP, PA and PP
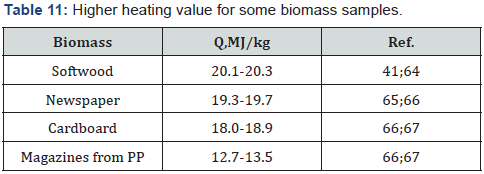
The obtained results are confirmed by literature data (Table
11).

Currently, around 400 million tons of paper and cardboard
(PB) are produced worldwide. However, after usage these
PB materials are thrown out and pollute the environment. In
economically developed countries such as the USA, Canada,
Western Europe, Japan and some others, about 50% of the waste
PB, mainly cardboard, is recycled. In Russia and China volume of
recycled PB is 20-30%. Unlike these countries, most Asian, African
and Latin America countries almost do not recycle the waste PB.
Thus, with an average of 75% of the waste BP or around 300
million tons in the world are land filled or burned.
As is known, the decomposition of PB and other organic waste
in dumps leads to emission of methane, which is recognized
20 times more dangerous greenhouse gas than carbon dioxide
[67,68]. Therefore, it is more preferable to burn the waste PB to
reduce the area of landfills, decrease CH4 emission and produce
heat or electricity [69]. For this purpose, it is necessary to know
energetic characteristics of the waste. The statistical analysis
showed that the average net combustion heat of paper trash
is 15 MJ/kg in the dry state [67], which corresponds to LHV of
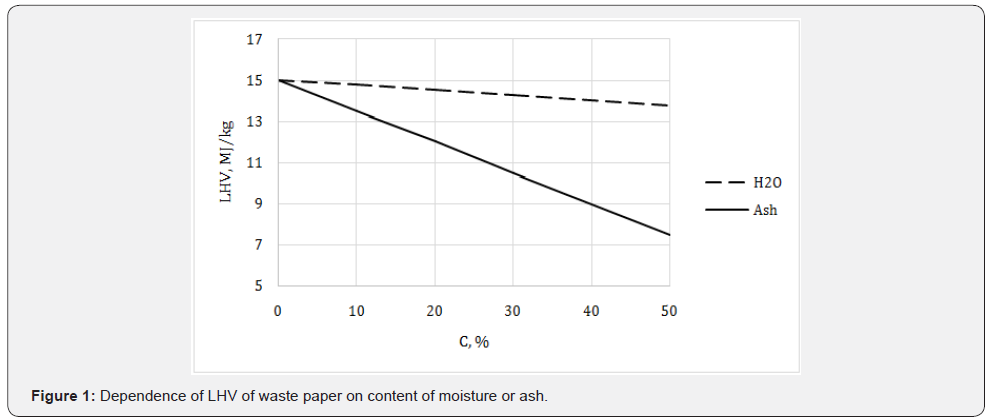
mixed waste paper (Table 10). In fact, a paper trash is always wet
and contains inorganic admixtures (ash); therefore its energy
potential is less than for dry and pure material (Figure 1).

An acute environmental problem is the accumulation of
synthetic polymers and their composites - plastics, which, unlike
biomass, are biostable and can persist in nature for hundreds of
years [70]. Every year in the world about 250-300 million tons
of synthetic polymers and plastics are manufactured [22]. Over
90% of all plastic products are made of rubber, polyethylene
(PE), polypropylene (PP), polystyrene (PS) and polyethylene
terephthalate (PET).Only part, 30-35%, of these waste, mainly
PET-bottles, rubber tires and some other polymeric articles, is
recycled in the world. But about 65-70% or 150-170 million tons
of biostable polymers and plastics are thrown away annually and
pollute the environment. The dumps occupy huge areas, which
are required for economy of countries. Moreover, in order to
equip the dump and keep it at the level of modern environmental
requirements, large funds are needed. Landfill sites are sources of
pollution of the environment with harmful substances contained
in plastics such as monomers, plasticizers, cross linkers, flame
retardants, etc. Particularly dangerous are small particles of
plastics. These pollutants poison the air, soil, water, and create a
danger to the vital activity of plants, animals and people.
A huge problem is the pollution of water bodies and oceans
with polymer debris [23]. Every year, up to 10 million tons of
plastics are thrown into the ocean. It was found that currently
more than 7 billion tons of biostable plastic waste have been
accumulated in the world. The cardinal way of destroying plastic
debris is burning. Despite CO2 emission, the modern technology of
burning (e.g. in plasmatrons) causes less harm to the environment
than the accumulation of huge volumes of biostable waste plastics.
Along with the eliminating plastic debris, this method allows to
receive an additional energy.
The specific thermal energy of synthetic polymers and plastics
can be determined experimentally using calorimetric bomb or
calculated by eq. (6), (9) or (13) (Table 2 & 4). Typical HHV and
LHV of the most spread synthetic polymers are shown in Table 12.

Thus, polyolefins (POL) and rubber (RUB) have higher
potential of heat energy than oxygen-, nitrogen- or halogencontaining
plastics (PMMA, PET, PA-6, PVC). The last four plastics
should be pulled out from plastic trash before combustion of POL,
RUB or mixture thereof.
As is known, waste of biomass and plastics have different
sizes, shapes and composition, as well as low bulk density. These
features of solid organic matters leads to unsatisfactory fuel
properties such as unstable calorific value, poor energetic density
and insufficient combustion efficiency [72,73]. For example, trash
of biomasscan have moisture from10 to 55%. Some biomass
residue or waste, e.g. rice straw or husk, packaging and printing
paper, contain from 15 to 30% of mineral components that
hinder combustion and reduce calorific value. The bulk density
of biomass varies from 40-80kg/m3 (grasses), to 150-200kg/m3
(wood chips), whereas the energy density is relative low, from 0.5
to 5GJ/m3. Plastics trash also contain pieces of various sizes and
shapes of different polymers with low bulk density, from 50 to
200kg/m3 that lead to low energy density, from 2 to 6GJ/m3. The
negative features of waste materials hinder their application as
solid fuels for the energy recovery. To overcome the low energetic
density and other negative fuel characteristics, the loose matters
should be converted into dense pellets or granules.
Pelletization of plant biomass is a well-known process, which
includes a number of steps: selection, removal of foreign pieces,
drying to a moisture content of 10% or less, shredding, grinding
to particle size of 1 to 3mm, hot pressing at 80-90oC into long rod,
cooling and cutting of the rod into pellets [73].The mineralized
and very wet materials must be previously removed at the
selection step. The pellets can be easily prepared from waste and
residues of various plant materials, but especially from feed stocks
having low moisture and ash content, as well as increased content
of lignin and lipids [1,74]. The raw material for the production of
granules is plastic waste. After disposal of PET bottles from trash,
the remained waste contains mainly polyolefins having average
HHV=42MJ/kg. Processing of plastic waste comprises steps of
removal of foreign materials, shredding, melting and granulating.
The prepared pellets and granules are strong and dense; they
have a typical bulk density of 600 -700kg/m3and energy density
of 10-12GJ/m3for biomass pellets and 20-30GJ/m3 for plastic
granules. Such densified solid fuels represent a promising source
of additional heat energy with improved burning productivity.
Besides, the compacted solid fuels are more economical to
transportation [75].
The granulation technology makes it possible to obtain fuel
granules with unique properties from mixed materials containing
both plastic and biomass waste [76]. The addition of plastic to
biomass reduces hydrophility and increases the strength, density
of energy and calorific value of the mixed solid fuel as compared
to biomass pellets only. Furthermore, since the plant biomass is
neutral for CO2- emission, burning of granules made from mixed
natural and synthetic materials results in a lower release of this
greenhouse gas in comparison with a separate burning of plastic
granules only.
Total amount of biomass aimed especially for energy
production is estimated at 7-8 billion tons with energetic potential
of 100-120EJ [77]. However, currently only half of this potential,
50-60EJ, is used to recovery energy from biomass, i.e. about
10%, of the global energy production [19]. Currently, biomass
is remained the main source of energy for population living in
rural areas of various countries, which uses firewood, chips,
sawdust, twigs, bushes, straw, and other plant residues as solid
biofuel to meet their energy needs. Share of biomass energy use
in developing counties is 75% and in developed counties is 25%
[77]. Besides, in economically developed countries the biomass
waste is burned as a part of MSW in order to eliminate solid
waste, reduce area of dumps, prevent environmental pollution
and generate additional energy [78]. Combined heat and power
systems that use the biomass-based fuel are widespread in
Europe and especially in the Scandinavian counties [79]. In North
America and European countries the thermal energy is generated
also by burning of waste from forest and paper industry, as well as
by burning of fuel pellets. Global production volume of the pellets
is about 30 million tons; moreover, the growth of the production
of such biofuel is expected to 50 million tons by 2020 [74].
As for plastics, it is known that over the last 70 years about
7 billion tons of wastes of various biostable plastics have been
accumulated in the world, not counting another 150-170 million
tons plastic waste that are thrown out every year. If gradually
incinerate this amount of waste for 10 years, about 24-25EJ of
thermal energy can be produced each year, whereas burning
of another 150-170 million tons of plastic waste gives 6-7EJ of
additional energy.
Thus, the total energetic potential of biomass and plastic
wastes is estimated at 140-150EJ per year, which can provide 22-
25% of the global energy production. Moreover, to produce the
thermal energy it is most promising to burn the granulated mixed
fuel containing biomass and additive of plastic waste.
Energy Potential of Liquid and Gaseous Fuels Extracted from Solid Polymer Materials
Solid polymer materials can be also converted into liquid and
gaseous fuels. For example, such known liquid fuel as ethanol
is produced from cellulose and various types of plant biomass.
Technology of cellulosic ethanol comprises three steps. The first
step is hydrolysis of cellulose with acids or cellulolytic enzymes
to obtain glucose:
(C6 H10 O5)n + nH2 O→nC6 H12 O6
The second step is fermentation of glucose into ethanol in the
presence of yeast:
C6 H12 O6→2C2 H5 OH + 2CO2
And the final stage is distillation and dehydration of ethanol.
The theoretical yield of ethanol is 51% from mass of glucose
and about 57% from mass of cellulose, but the actual yield is lower.
A more difficult task is to obtain ethanol from biomass.
This is due to the dense structure of plant materials and barrier
properties of lignin, which hinder the diffusion of hydrolyzing
reagents - acid or enzyme [80,81]. Therefore, a pretreatment
step is applied to make the biomass more accessible to reagents.
The main objectives of pretreatment are to loosen the physical
structure of biomass and to eliminate the lignin in order to
increase accessibility of biomass and the percentage of cellulose
fraction. Various pretreatment methods of lingo cellulosic
biomass can be used such as steam explosion, acidic treatment,
alkaline extraction, oxidation, organosolv and some others [81-
84]. Among various methods, alkaline pretreatment of plant
biomasses under mild conditions is intensively studied due to
their industrial feasibility, relatively low capital investment, as
well as low consumption of chemicals and energy [83-85]. Twostep
pretreatment using combinations of acid and alkali are
considered as very effective [86,87].
Hydrolysis step of cellulose in pretreated biomass is
usually
carried out by cocktail of cellulolytic enzymes, which provide a
higher yield of glucose than acid hydrolysis. This cocktail consists of
three main enzyme types, endoglucanase, exo-glucanase and
beta-glycosidase, acting synergistically [80].
Known types of enzymatic cocktails are CellicCTec 2 and
CellicCTec 3 of Novozymes, as well as Accellerase-1500 and GC-
220 of DuPont/Genencor. Optimal conditions of the enzymatic
hydrolysis are the following: temperature is 45 to 50 °C, pH = 4.5
to 5.0, dose of enzymes is 10 to 20mg per 1g of solid biomass and
loading of biomass substrate is 140-160g/L.
The fermentation of glucose solution obtained after enzymatic
hydrolysis of the pretreated biomass is carried out usually by the
yeast of Saccharomyces cerevisiae in the presence of nutrients at
30 °C and pH=5 [88].Various fermentation methods can be used:
simultaneous saccharification and fermentation, simultaneous
saccharification and co-fermentation, sequential fermentation,
continuous fermentation, etc. The final concentration of ethanol
after fermentation step is usually 50-60g/L. Therefore the dilute
“beer” is distilled to obtain 95-96% of ethanol. Since the fuel
ethanol should not contain more than 1% water, the distilled
ethanol is dehydrated to 99% using dewatering agents, e.g. zeolites.
The yield of ethanol at the same saccharification and fermentation
conditions depends on type of biomass and pretreatment method.
For example, yield of ethanol from alkali pretreated corn stover is
30-35% that is 18-21% from initial biomass.
Hydrolyzate of cellulose or pretreated biomass containing
glucose can be fermented also by oleaginous microorganisms for
lipid production. They are yeasts of Rhorosporidiumtoruloides,
Lipomycesstarkeyi, Mortierellaisabellina, Trichosporonfermentans,
Cryptococcus curvatus etc. [89,90]. The fermentation of glucose
solution with oleaginous yeasts was carried out at 30oC while
stirring for 4-7 days. As results, from 20 to 50% of glucose are
converted to lipids, which further can be uses for production of
biodiesel fuel [91,92].
The technology of biodiesel fuel is based on transesterification
reaction between lipid and alcohol, e.g. methanol, in the presence
of alkaline or acid catalysts [93, 94]:
C3 H5 (O2 CR)3 + 3CH3 OH →3RCO2 CH3 +C3 H5 (OH)3
The obtained ester is used as a biodiesel fuel, and the
byproduct, glycerol, can be utilized for some other applications.
Anaerobic digestion of biomass allows to obtain biogas
such as methane. The most appropriate feed stocks for biogas
production are waste paper or residue of pulp and paper industry.
Lignocellulosic biomass can be pretreated to loosen the physical
structure of substrate and reduce content of lignin hindering the
bioconversion process [95]. The dilute aqueous dispersion of
substrate (5-10%) is digested in a bioreactor under anaerobic
conditions at optimal pH of 6.8-7.4 under the action of mesophilic
bacteria at temperature of 30-40 °C or thermophilic bacteria at
temperature of 50-60 °C. The source of these bacteria can be
sludge of sewage or cattle manure. The process of anaerobic
biodegradation usually takes 2.5 to 3 weeks.
The process of anaerobic biodegradation of organic substances
occurs in several stages [96]. In the first stage, hydrolysis, the
complex molecules (СM) of organic substances break down into
simple molecules (SM). In the second stage, acetogenesis, simple
molecules produced in the first stage are converted to acetic acid
(AA). In the last stage, methanogenesis, acetic acid is converted to
biogas (BG) containing methane, carbon dioxide and some other
gases.
CM →SM → AA→ BG
For example, cellulose and other C6-polysacharides (C6 PS)
are hydrolyzed into monomeric glucose (GL). Further, glucose
forms acetic acid, which is transformed into biogas.
C6 PS →GL → AA → BG
(1). C6 H10 O5 + H2 O→C6 H12 O6
(2). C6 H12 O6 →3CH3 COOH
(3). 3CH3 COOH →3CH4 + 3CO2
Total process: C6 H10 O5 + H2 O→3CH4 + 3CO2
The anaerobic digestion of xylan and others C5-polysacharides
(pentosans) can be described as follows:
(1). C5 H8 O4 + H2 O→C5 H10 O5
(2). C5 H10 O5 →2.5CH3 COOH
(3). 2.5CH3 COOH →2.5CH4 + 2.5CO
Total process: C5 H8 O4 + H2 O→2.5CH + 2.5CO2
The theoretical yield of biogas ( YYg ), containing mixture of CH4
and CO2, and methane only (
Ym ) from 1 t of dry organic substance
(DOS) with molecular weight, M (g/mole), can be calculated by
the equations [97]:

Where m and k is number of moles of CH4 and CO2, respectively,
formed from 1 mole of DOS.
Using Eq (16) and (17) it can be calculated that the theoretical
yield of biogas from 1 t of cellulose and other C6 PS is 830 m3
and of methane is 415m3. In the case of pentosans the theoretical
yield of biogas is 848m3, whereas of methane is 424m3 from 1 t
of DOS. Maximum yield of biogas from 1 t of biomass, containing
65% of polysaccharides (PS), is about 546m3 and of methane
273m3.However, the actual yield of biogas and methane from
plant polymers and biomass for real time of bioconversion is
significantly lower. For example, bacterial digestion of 1 t wheat
straw with 65% content for 3 weeks of polysaccharides results in
the production of about 150m3 methane, i.e. 55% from theoretical
yield.
Biomass of microalgae is promising raw-material for
bioenergy recovery. Microalgae (MA) include variety of species such as Ankistrodesmus, Botryococcus. Chlorella, Nannochloropsis,
Neochloris, Nitzschia, Scenedesmus, Schizochytrium etc. The
peculiarity of MA is that they contain lipids. Among them
Schizochytrium, Nannochloropsis and Botryococcus contain
particularly high amount of lipids up to 70-75% [98]. Extracted
lipids are used for production of biodiesel fuel through
transesterification reaction [99,100]. The residual biomass of MA
remaining after extraction of lipids can be burned or subjected to
anaerobic digestion to produce biogas [101].
Liquid and gaseous fuels are obtained also by pyrolysis of
biomass or plastics. Biomass wastes and residues are subjected
usually for this purpose. The main products of the pyrolysis
are bio-oil, gases and charcoal [102,103]. The yield of various
products is dependent on conditions of the pyrolysis process and
biomass composition. Slow pyrolysis of the biomass is carried
out at heating rate ≤1degree per sec up to 400-500 °C and typical
residence time of 10 to 20min with formation of 20 to 40% biooil,
30 to 50% gases and 25 to 35% charcoal. If the pyrolysis
temperature is above 500 °C, a decrease in the yield of charcoal
is observed. Slow pyrolysis of biomass at high temperatures, 800
to 1000 °C in the inert or steam atmosphere causes the formation
mainly gaseous product containing CO2, CO, H2, CH4 and some
other gases with calorific value 10- 12MJ/Nm3or 14-16MJ/kg
[104].
Fast pyrolysis is performed at heating rate 100-200degrees
per sec, temperature above 800 °C and short residence time
especially to increase the yield of bio-oil. Flash pyrolysis at heating
rate above 1000 degrees per sec and temperature above 1000 °C
allows obtain the bio-oil with a high yield of 70-75%. The calorific
value of the bio-oil is 15-17MJ/kg, which is significantly lower
than of ethanol, biodiesel and hydrocarbon fuels [1]. Pyrolysis
method is used also for decomposition of waste plastics to obtain
combustible liquids–pyrolytic hydrocarbons (HC) [105,106].
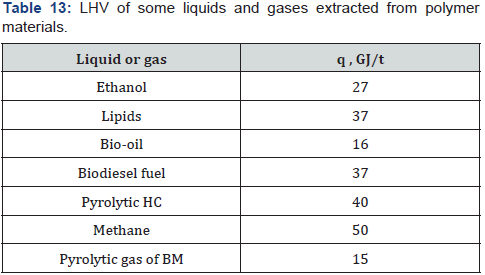
Calorific values of various liquid and gases extracted from biomass
or plastics are shown in Table 13.

These values were used to calculate the energy yield (EY):

Where Y is yield of liquid or gas (t) obtained from 1 t of initial
polymer material; q is LHV of liquid or gas (GJ/t), and qo is LHV of
initial polymer material (GJ/t)
Knowledge of the energy yield allows to understand that it
is more advantageous, the direct burning of the starting polymer
material (biomass or plastic), or the burning of various liquid or
gaseous fuels extracted from this material? The typical examples
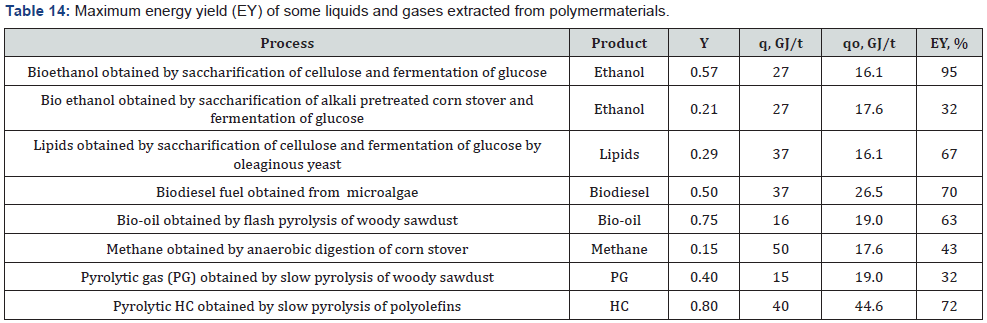
of energy recovery are presented in Table 14.
Analysis of these typical examples reveals that in all cases
the energy yield of liquid or gaseous products extracted from 1
t of starting materials (biomass or plastic) is significantly lower
than the thermal energy of 1 t of the starting materials (Table 14),
(Figure 2).


Thus, to generate the thermal energy, it is more profitable to
burn the starting material as solid fuel, than extracted liquid or
gaseous fuels.
Conclusion
The energy potential of natural and synthetic polymers, plant
biomass, plastics and their solid waste, as well as combustible
liquids and gases derived from them has been studied. To
determine calorific values of solid, liquid and gaseous substances
various experimental and calculation methods were used. For this
purpose, improved equations based on chemical structure and
elemental analysis was proposed. It was shown that conversion of
solid materials into liquids or gases decreases the yield of thermal
energy. Therefore for the production of thermal energy, it is more
profitable to burn the solid biomass or plastic than combustible
liquids and gases, derived from them. Studies have shown that
lipids and lignin increase, whereas moisture and ash reduce the
calorific value of biomass. The calorific value of most synthetic
polymers and plastics is higher than that of biomass samples, but
resources of waste plastics are about 10 times lower than these of
biomass. It is most promising to burn the granulated mixed fuel
containing biomass and about 10% additive of plastic waste, since
it enables to increase calorific value and energy density of biomass,
as well as to reduce emission of carbon dioxide in comparison
with separate incineration of plastic only. Considering the total
amount of biomass and plastics waste destined for combustion,
it was calculated that current annual energy potential of biomass
is about 115EJ, and of plastic waste about 30EJ, which can cover
together about 24% of the world’s annual energy production. The
problem is that plastics are produced from fossil raw materials,
whose reserves are not sustainable and permanently depleted. In
this regard, the wider use of biomass has a significant advantage
because their reserves in nature are huge and continuously
renewed. In the near future, it is planned to increase the share
of biomass in the production of alternative energy twice at
least, which can simultaneously contribute to reducing in green
house gas emission and improving the ecological state of the
environment.
For more
details Academic
Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
To read more…Full Text in in Juniper
Publishers click on https://juniperpublishers.com/ajop/AJOP.MS.ID.555553.php


Comments
Post a Comment